Fluorine Notes, 2002, 25, 1-2
The electrolytic method of fluorination in the medium containing the complexes of anhydrous hydrogen fluoride and trialkylamines.
G.G.Furin
Novosibirsk Institute of organic chemistry named after N.N.Vorozhtsov
Siberian branch of the Academy of Science of Russian Federataion
Fax: +7-3822-344752
e-mail: furin@nioch.nsc.ru
ABSTRACT
The review generalizes and systematizes the latest date on fluorination of the various classes of organic compounds by the electrolytic anodic oxidation method in the medium of electrolyte the complexes of anhydrous hydrogen fluoride and trialkylamines. An analysis was performed of the basic achievements of the method of one atom fluorine introduction. The facts having an influence on the process and influence of electron-seeking substitutes arranged by CH-fragment and by heteroatoms of VIA group are shown. The examples are given of the useful applications of this method for the synthesis of aromatic and heterocyclic compounds as well as compounds having XCHF fragments, where X=S, Se, Te, as the potential intermediate products for synthesis of the preparations possessing a biological activity. The new efficient production methods of monofluorine containing organic compounds and their role in organic synthesis are discussed.
Contents
Introduction
1. Electrolytic fluorination of organic compounds in the presence of the electrolyte containing the complexes of anhydrous hydrogen fluoride and trialkylamines.
1.1 Anodic monofluorination of aromatic compounds
1.2 Electrolytic fluorination of heterocyclic compounds.
1.3. Anodic fluorination of the organic compound with CH-fragment containing electron-seeking substitutes.
2. Behavior of organic derivatives of the VI group elements, having CH-fragment in alpha position to heteroatom in the electrolytic solutions containing hydrogen fluoride.
Conclusions
References
1.2. Electrolytic fluorination of heterocyclic compounds
Partly fluorinated heterocyclic compounds are in focus of researchers due to their high biological activity. They are used as intermediate products when creating preparations for medicine and agriculture. Their synthesis is rather non-selective and limited in many cases. Continuous search for fluorinating reagents for introduction of the fluorine atoms and reagents for introduction of perfluoroalkyl groups into organic molecules [56,57] is going on because of this. Unlike this electrochemical fluorination method is the ideal method of direct fluorination and can be carried out at one go when conditions are mild [58]. However there are few examples of such fluorination, though high yield and selectivity make this method rather attractive [34,43,59-61].
Having a carbonyl group in 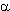 -position to nitrogen atom N-methylpyrazoles are subject to electrolytic fluorination in the Py/9HF system at 20 oC with formation of fluoropyridizinone [62].Et3N*3HF system can also be used as electrolyte [63].
-position to nitrogen atom N-methylpyrazoles are subject to electrolytic fluorination in the Py/9HF system at 20 oC with formation of fluoropyridizinone [62].Et3N*3HF system can also be used as electrolyte [63].
In the case of 4-thiazolydinone 10 Et3N*3HF/MeCN system proved to be rather effective and convenient. The formation of monofluoroderivative 11 with high regioselectivity occurs when carrying out anodic fluorination. These are the first examples of effective anodic fluorination of sulfur-containing heterocyclic compounds [64,65]. Subsequent oxidization using peracids affected sulfur atom and compound 12was formed.
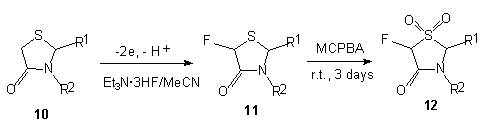
| R1 | R2 | Yield 11, % | cis:trans | Yield 12, % | cis:trans |
| 1-naphthyl | Me | 79 | 29:71 | ||
| Ph | H | 14 | 35:65 | ||
| Ph | Me | 42 | 35:65 | ||
| Ph | CMe3 | 59 | 45:55 | ||
| Ph | Ph | 84 | 43:57 | ||
| PhCH2 | Ph | 65 | 46:54 |
Other heterocyclic compounds react similarly.
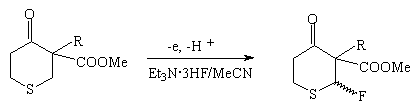
| R | Yield,% | Stereoselectivity,% |
| Me | 7 | 100 |
| CH2Ph | 27 | 76 |
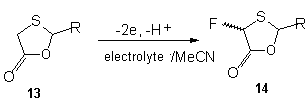
So, during anodic fluorination of 1,3-dithiolane-4-ones [66] and 1,3-oxathiolan-5-ones 13, which have the substitutes in position 2, appropriate fluoroderivatives are formed [67].
In table 4 there is information on anodic fluorination of 2-substituted 1,3-oxothiolane-5-ones, during which monofluoroderivative 14 [67] is formed. We'll notice, that fluoride-ion matters a lot in this process.
Table 4.Anodic monofluorination of 2-substituted 1,3-oxothiolane-5-ones [67].
| R | Eoxp/B vs SSSE* | Electrolyte | Potential of oxidation, V | F/mol | Yield of 14, % | cis/trans |
| Et | 2.34 | Et3N*3HF Et4NF*4HF | 2,3 2,1 | 3,4 2,6 | 0 86 | 47/53 |
| n-Pr | 2,32 | Et3N*3HF Et4NF*4HF | 2,2-2,3 2,1 | 3,6 2,2 | 5,4 67 | d 45/55 |
| i-Pr | 2,44 | Et3N*3HF Et4NF*4HF | 2,2 2,1 | 2,2 2,4 | 0 78 | 43/57 |
| Ph | 1,88 | Et3N*3HF Et4NF*4HF | 1,6 2,0 | 4,0 2,3 | 0 70 | 45/55 |
| 4-CNC6H4 | 2,17 | Et3N*3HF Et4NF*4HF | 2,2 2,0 | 3,4 3,2 | 0 66 | 39/61 |
* Pt- electrodes, 0,1 M NaClO4/MeCN
Taking into attention the data listed in the table 4 the system Et3N*3HF is unusable for carrying out of the anodic fluorination processes for this heterocycle.
4-Arylthio-1,3-dioxalane-2-one in dimethoxyethane undergoes the fluorodesulfurization and fluorination with formation of 4-fluoro-1,3-dioxolane-2-one [68].
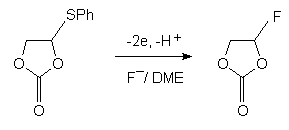
Electrochemical oxidization of 2,2,-diphenyl-1,3-dithiane in Et3N*4HF system results in formation of difluorodiphenylmethane [69].
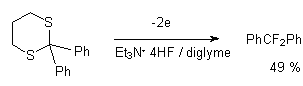
Monofluorinated 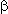 -lactams are of interest of fluorocontaining antibiotics synthesis as intermediate products and they also are of interest of creating amino-acids and sugars as block. Thus, regioselective anodic monofluorination of 3-phenylthio-2-azetidinones and 4-phenylthio-2-azetidinones results in formation of 3-and 4-substituted derivatives of 2-azetidinones[60]. The product yield of monofluorination essentially depends on electrolyte used. It is interesting that in case Py/(HF)n system doesn't really work [70].
-lactams are of interest of fluorocontaining antibiotics synthesis as intermediate products and they also are of interest of creating amino-acids and sugars as block. Thus, regioselective anodic monofluorination of 3-phenylthio-2-azetidinones and 4-phenylthio-2-azetidinones results in formation of 3-and 4-substituted derivatives of 2-azetidinones[60]. The product yield of monofluorination essentially depends on electrolyte used. It is interesting that in case Py/(HF)n system doesn't really work [70].
| R | Electrolyte | B, vs. SCE | F/mol | Yield,% |
| Et | 2,5 | 65 | ||
| Pri | Et3N . 3HF | 1,8 1,5 1,9 1,7 | 2,3 2,0 2,2 13,2 | 77 64 traces 33 |
| Bun | 1,8 | 2,4 | 92 | |
| But | 1,9 | 2,5 | 67 | |
| c-C6H11 | 1,8 | 2,5 | 84 | |
| PhCH2 | 2,0 | 4,0 | 68 |
In general case electrochemical fluorination of N-substituted lactams in electrolyte Et3N*3HF/MeCN goes selectively and affects the carbon atom, being in 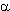 -position to nitrogen atom of lactam. In this case the monofluoroderivative product was formed, moreover the yield is rather high [71].
-position to nitrogen atom of lactam. In this case the monofluoroderivative product was formed, moreover the yield is rather high [71].
If SR group is not in 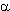 -position to C=O heterocycle group, then during anodic fluorination its replacement for fluorine atom occurs [72].
-position to C=O heterocycle group, then during anodic fluorination its replacement for fluorine atom occurs [72].
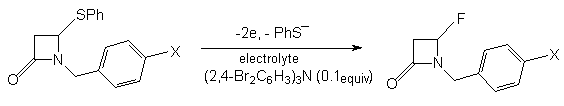
| X | Electrolyte | Yield,% |
| H | Et3N . 3HF/MeCN | 83 |
| Me | Et3N . 3HF/MeCN | 71 |
| Br | Et3N . 3HF/MeCN | quantitatively |
| H | Et4NF . 3HF/MeCN | 43 |
The presence of other substituents in heterocyclic ring doesn't change the reaction direction - fluorodesulfurization occurs[72].
The authors of article [53a] represent the mechanism of this process as follows.
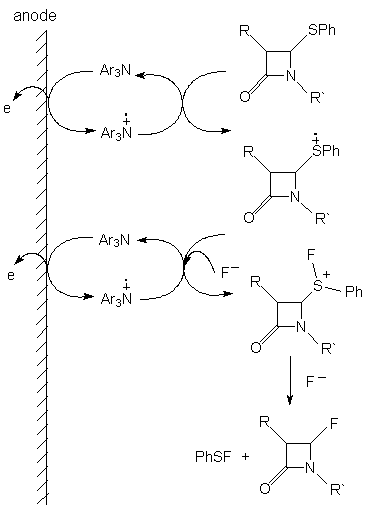
If in position 4 of azetidine-2-one there is SiMe3 group, then the formation of 4-substituted fluoroderivative also takes place (table. 5 ) [73].
Heterocyclic compounds with carbonyl groups are subject to selective fluorination too. For example, the formation of mono- and difluoro- derivatives of (4-methoxyphenyl)acetone, ethyl(4-methoxyphenyl)acetate [74,75] and fluoroderivatives of indan [76] is observed when carrying out anodic fluorination of hydrocarbon substrate in electrolyte-complex Et3N*3HF. Anodic fluorination of 1-naphthalene derivatives in Et3N*3HF system, oxyindole and 3-oxy-1,2,3,4-tetrahydroisoquinoline in Et4NF .3HF system goes without problems when groups CN, COOEt or SPh [77,78] are available.
Table 5. Electrochemical obtaining of 4-fluoroazetidine-2-one derivatives [73].
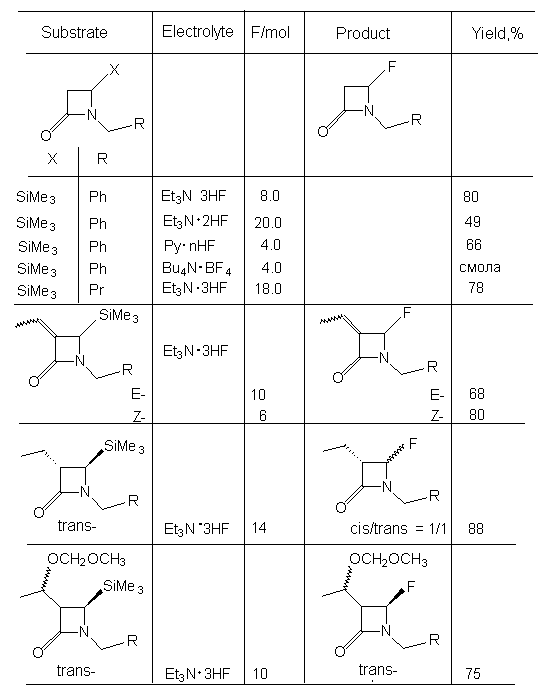
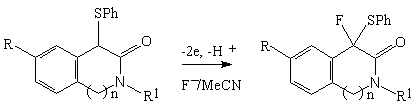
n R R1 Electrolyte F/mol Yield,% 0 H Ph Me4NF*4HF 3,5 64 0 Me 4-MeC6H4 Me4NF*4HF 3 50 1 H Ph Et4NF*3HF 2,6 71 1 H PhCH2 Et4NF*3HF 2 70
The nature of electrodes matters within certain limits. The increase of anhydrous hydrogen fluoride results in increment of fluorination product. As example, it was shown by anodic fluorination of oxyindole [77,78].
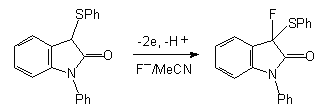
Anode | Yield,% | |
Et4NF*3HF | Me4NF*4HF | |
| Pt | 58 | 64 |
| Carbon | 28 | 60 |
| C-steel | 36 | 59 |
| C-felt | 21 | 42 |
At the same time anodic fluorination of oxyindole in Et4NF*3HF/MeCN system with absence of SPh group results in fluorination of benzene ring without affecting of heterocyclic system [79].
This example shows the importance of SPh group in anodic fluorination of different derivatives.
During anodic fluorination of ethyl isonicotinate [80], pyrazole (table 6) [61,81], pyridine [60,82], 1,10-phenanthroline [42,83,84] the fluorination products containing fluorine in heterocycle and in methyl group were formed. However the yield of fluoroderivatives is not very high.
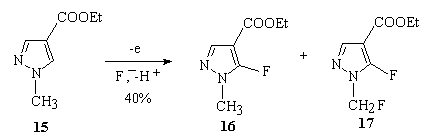
It was shown [61], that during electrochemical fluorination of 1-methylpyrazole-4-carboxylic acid ethyl ester 15 the using of 70 % HF/Py/Et3N complex on platinum anode results in formation of 1-methyl-5-fluoropyrazole-4-carboxylic acid ethyl ester 16 and 1-fluoromethyl-5-fluoropyrazole-4-carboxylic acid ethyl ester 17. The highest yield was 40 % and selectivity of fluorine attack in position 5 was 83% .
Anodic fluorination of pyridinein Et3N*3HF system gives 2-fluoropyridine with 22 % yield [60]. Heterocyclic ring containing electron-seeking substituents complicate the direct fluorination of cycle and the yield of 2-fluorosubstituted derivative is low [80]. For example, 2-fluoro-iso-nicotinic acid ethyl ester with high conversion is obtained using electrolysis of iso-nicotinic acid ester in Et3N*3HF/MeCN system with 30 % yield and 76% conversion [80].
Table 6.Anodic fluorination of 2-methylpyrazole-4-carboxylic acid ethyl ester [61].
| Anode | System | Solvent | Voltage, V | Products 16:17 ratio | Yield,% |
| Pt | Pt HF/Py | MeCN | 2,5 | 25:75 | 16 |
| HF/Py/Et3N(0.3) | 57:43 | 23 | |||
| HF/Py/Et3N(0.6) | 83:17 | 40 | |||
| HF/Py/Et3N(1.0) | 3 | 100:0 | 18 | ||
| HF/Py/Et3N(0.6) | THF | 2,7 | 100:0 | 2 | |
| HF/Py/Et2NPh | MeCN | 2,5 | 92:8 | 39 | |
| C | HF/Py/Et3N | MeCN | 2,1 | 97:3 | 31 |
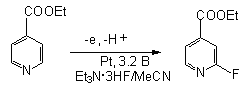
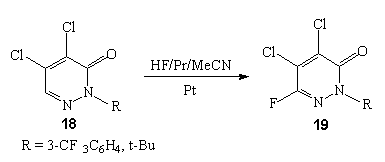
Nitrogen-containing heterocyclic compounds in electrolytes mentioned are subject to fluorination and as a rule hydrogen atoms are displaced in  -position to nitrogen atom of the cycle. For example, anodic fluorination of pyridazininone-3-onaderivative 18 in the medium 70 % HF/Py/MeCN at Pt electrodes at room temperature gives monofluoroderivative 19 [85].
-position to nitrogen atom of the cycle. For example, anodic fluorination of pyridazininone-3-onaderivative 18 in the medium 70 % HF/Py/MeCN at Pt electrodes at room temperature gives monofluoroderivative 19 [85].
Anodic fluorination of 2H-1,4-benzothiazine-3(4)-ones derivatives 20 in acetonitrile in the presence of Et3N*3HF results in formation of monofluoroderivatives 21, moreover fluorine displaces hydrogen in 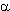 -position to sulfur atom of ring [86].
-position to sulfur atom of ring [86].
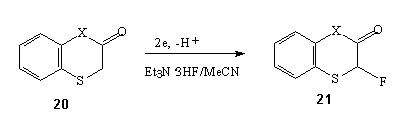
| X | Potential of anode, V | F/mol | Yield of 21,% |
| NH | 3.0 | 15 | 0 |
| NHCOPh | 2.0 | 2.2 | 77 |
| N-Pri | 1.5 | 2.5 | 88 |
| n-Me | 1.6 | 2.1 | 68 |
Anodic fluorination of biologically active flavone derivatives, having first potential Epox within 2.36 - 2.52 V, results in formation of difluoro- and trifluoroderivatives [84].
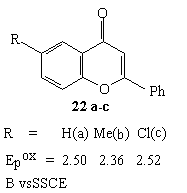
Anodic fluorination of flavone and 6-chloroflavone in Et3N *3HF and Et4NF*4HF systems mainly produced 3-monofluoroderivative with small quantity of 2,3-difluoroderivative. The process was carried out at 30 oC [27,87] and the ratio of products in reaction mixture depended on electrolyte used [84].
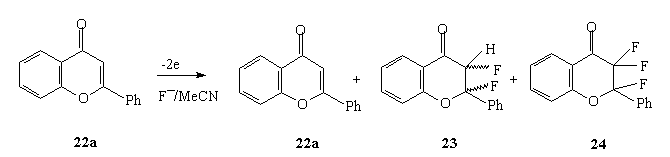
| Electrolyte | Yield, % | ||
| 22a | 23 (cis:trans) | 24 | |
| Et3N.3HF | 43 | 0 | 6 |
| Et3N.5HF | 9 | 4 | |
| Et4NF.3HF | 3 | 54(2:1) | 6 |
| Et4NF.4HF | 7 | 68(2:1) | 9 |
At that the temperature has a key influence:
| Temperature, oC | -10 | 0 | 10 | 20 | 30 |
| Yield 22a,% | 9 | 27 | 31 | 43 | 58 |
| Yield 24,% | traces | 3 | 4 | 6 | 19 |
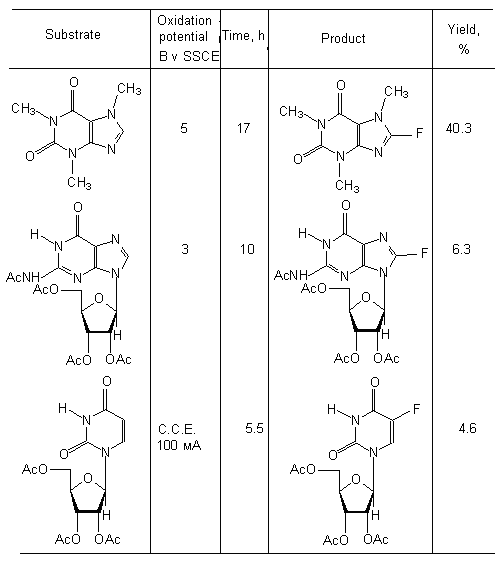
Electrochemical oxidization of caffeine, guanosine tetra-acetate and uridine tri-acetate, proceeding in electrolyte Et3N . 3HF, results in formation of monofluoroderivatives with small yield (table 7) [88].
It should be noted that there is the high regioselectivity during anodic fluorination of 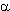 -phenylsulpho phenyl substituted lactams 25 in Et3N .3HF/MeCN medium (Pt electrodes) when hydrogen is substituted by fluorine in
-phenylsulpho phenyl substituted lactams 25 in Et3N .3HF/MeCN medium (Pt electrodes) when hydrogen is substituted by fluorine in 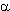 -position to Sph-group independently of ring size 26 [86].
-position to Sph-group independently of ring size 26 [86].
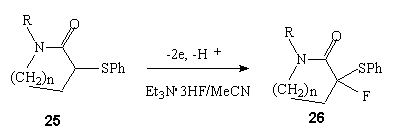
| n | R | Potential of anode (V), relative to SSCE | F/mol | Yield of 26,%, |
| 1 | Me | 1,8 | 2,5 | 85 |
| 1 | cyclohexyl | 1,8 | 2,5 | 84 |
| 2 | Me | 2,0 | 2,2 | 69 |
| 3 | Me | 1,9 | 4,0 | compounds mixture |
1.3. Anodic fluorination of the organic compound with CH-fragment containing electron-seeking substitutes.
High yield of fluoroderivatives is achieved by anodic fluorination of substituted derivatives both aromatic and heterocyclic compounds, having protons movable enough in 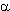 -position to the main cycle. As a rule, most effective Et3N* 3HF system and platinum electrode are used [89,90].
-position to the main cycle. As a rule, most effective Et3N* 3HF system and platinum electrode are used [89,90].
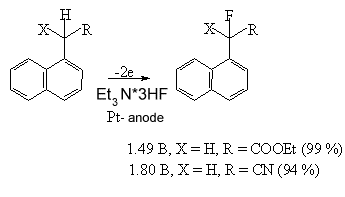
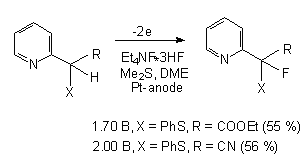
Table 7.Electrochemical oxidization of caffeine and similar compounds (MeCN, Et3N * 3HF) [88].
If in benzene ring there are electron-donating substituents, then during anodic fluorination active methene group of substituent will be affected mostly [75,91-94].
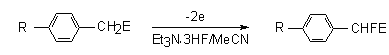
| R | E | Potential, V | Conversion | Yield,% |
| MeO | COMe | 1,2 | 100 | 72 |
| MeO | COC6H4OMe | 1,2 | 99 | 72 |
| Cl | COOEt | 1,7 | 75 | 36 |
| MeO | COOEt | 1,28 | 100 | 69 |
| 3,4(MeO)2 | COOEt | 0,8 | 92 | 73 |
| CH2=CHCH2O | COOEt | 1,49 | 100 | 51 |
| H | CN | 2,33 | 44 | 22 |
| MeO | SO3Et | 1,37 | 96 | 71 |
| Cl | CN | 1,88 | 65 | 64 |
| MeO | CN | 1,37 | 97 | 67 |
It is shown, that the reaction goes via initial generation of cation-radical of substituent atom neighboring to -CH2- fragment with following fluoride-ion attack of cationoid centre of intermediate carbcation, which is in 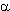 -position to carbonyl group [92,94].
-position to carbonyl group [92,94].
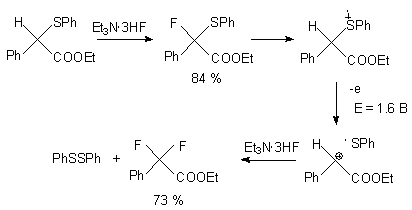
Anodic fluorination of sulfides in Et3N*3HF medium results in formation of monofluoro- and hem-difluoroderivatives [95]. At that elimination of SPh group occurs .
Anodic oxidization of benzylalkyl ketones, carboxylic acids esters, nitriles, benzyl derivatives in the system Et3N*3HF/MeCN at platinum anode enables to obtain monofluoroderivatives, some of which are biologically active [64,70,91,96-100].

Substituent in benzene ring influences greatly on the ratio of resulting products [91].
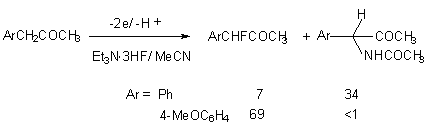
At the same time electrophilic substituents in 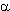 -position to methene group have a light influence on the anodic fluorination process, while the increasing of voltage results in increasing of difluoro-derivative yield.
-position to methene group have a light influence on the anodic fluorination process, while the increasing of voltage results in increasing of difluoro-derivative yield.
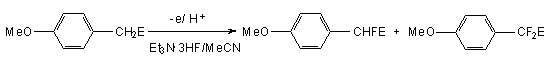
| E | Voltage,V | Yield,% | Voltage,V | Yield,% |
| CH3CO | 1,2 | 69 | 1,6 | 61 |
| 4-MeOC6H4CO | 1,2 | 71 | 1,55 | 50 |
| COOEt | 1,28 | 69 | 1,45 | 55 |
| CN | 1,35 | 65 | 1,60 | 50 |
The presence of methyl group in benzene ring results in its fluorination. Thus, p-methylbenzylsulfonate during anodic fluorination in Et3N*3HF/MeCN system forms the mixture of electrolysis products 27-30 [93].
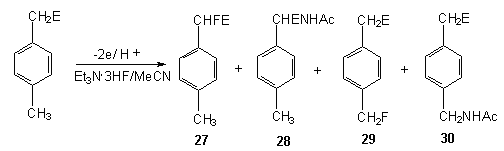
| E | Voltage, V | Yield,% | |||
| 27 | 28 | 29 | 30 | ||
| COOEt | 1,65 | 40 | 14 | 8 | 18 |
| CN | 1,73 | 58 | <1 | 3 | 6 |
| SO3Et | 1,78 | 15 | 0 | 21 | 30 |
| H | 1,80 | 10 | 25 |
Electrochemical oxidization of aliphatic aldehydes in Et3N*5HF or Py* nHF (n = 3-6) in acetonitrile or sulpholane results in formation of corresponding carboxylic acids fluoroanhydrides with high yield [101,102].
2,2-disubstituted cyclic ketones in Et4N*5HF at anodic fluorination produce fluoroanhydrides of appropriate fluorocontaining carboxylic acids due to selective splitting of С-С bond between carbonyl atom of carbon and 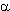 -substituted carbon atom (table 8) [103-105]. Thus, electrolysis of 2,2-dimethylcyclohexanone in electrolyte Et4N*5HF at 0oC with subsequent addition of methyl alcohol produces methyl 6-fluoro-6-methylheptanoic acid ester.
-substituted carbon atom (table 8) [103-105]. Thus, electrolysis of 2,2-dimethylcyclohexanone in electrolyte Et4N*5HF at 0oC with subsequent addition of methyl alcohol produces methyl 6-fluoro-6-methylheptanoic acid ester.
Probably, the reaction path is the following:

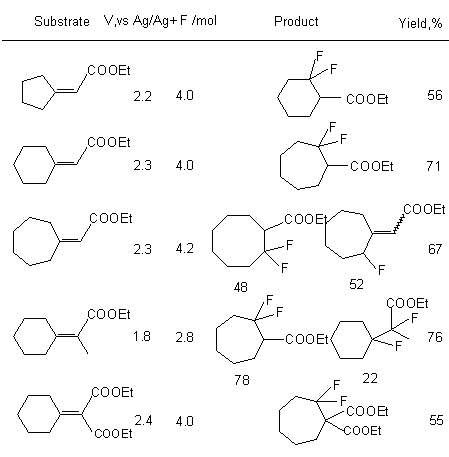
At the same time anodic fluorination of unsaturated carboxylic acids esters having cylcopentane and cyclohexane fragments in Et3N*5HF system results in forming of expanded by one carbon atom cycle. 2-2-Difluorocycloalkanecarboxylic acids esters with high selectivity and good yield are produced (table. 9) [106,107].
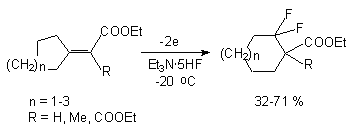
Table 8. Synthesis of carboxylic acids esters using electrosynthesis (electrolyte Et3N*5HF, 0oC) of cyclic ketones [103].
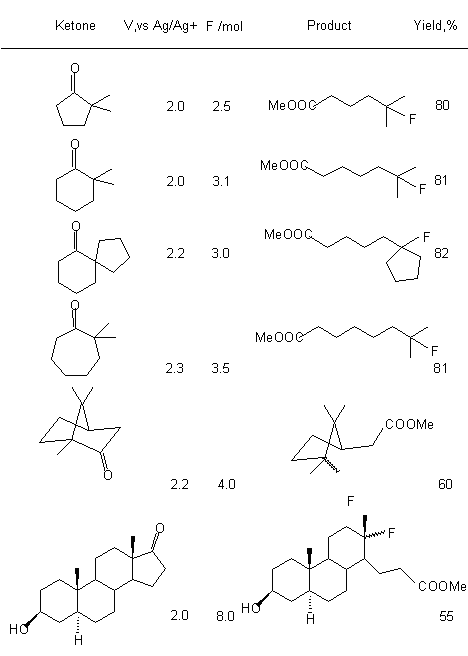
Table 9. Electrochemical oxidization of unsaturated cyclic esters [103].
Anodic fluorination of different N-substituted lactams using electrolyte Et3N*3HF results in formation of corresponding monofluorocontaining products, in which fluorine atom is in  -position to nitrogen atom [105].
-position to nitrogen atom [105].
During electrolysis of 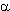 ,
,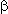 -unsaturated esters thehem-
-unsaturated esters thehem-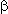 ,
,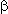 -difluoroderivatives of esters were formed in electrolyte due to rearrangement of alkyl group from
-difluoroderivatives of esters were formed in electrolyte due to rearrangement of alkyl group from 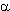 to
to 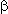 -position [107]. If alkenyl group is in this position, for example in case of carboxylic acids esters of dienes , then the fluorination with formation of vicinal difluoroderivatives will occur (table 10) [102].
-position [107]. If alkenyl group is in this position, for example in case of carboxylic acids esters of dienes , then the fluorination with formation of vicinal difluoroderivatives will occur (table 10) [102].
Table 10.Anodic fluorination of dienes esters [102].
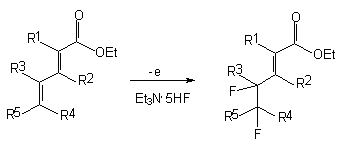
| R1 | R2 | R3 | R4 | R5 | Electrolyte | F/mol | Potential of anode, V | Yield, % | Isomer ratio |
| H | H | H | Me | Me | Et3N*5HF | 2,5 | 1,6 | 73 | - |
| H | H | H | H | Ph | Et4NF*2HF | 2,5 | 1,4 | 35 | 2,5:1 |
| H | H | H | H | Me | Et3N*5HF | 2,5 | 1,8 | 69 | 2:1 |
| H | H | H | H | n-C6H13 | Et3N*5HF | 2,5 | 1,8 | 53 | 5:1 |
| H | H | Me | H | Me | Et3N*5HF | 2,5 | 1,6 | 61 | 1,5:1 |
| H | Me | H | Me | Me | Et3N*5HF | 2,5 | 1,6 | 16 | - |
| H | Me | H | Me | Me | Et3N*3HF | 8 | 1,6 | 45 | - |
| Me | H | H | Me | Me | Et3N*5HF | 2,5 | 1,25 | 86 | |
| COOEt | H | H | Me | Me | Et3N*5HF | 2,5 | 1,6 | 85 | 3:1 |
| COOEt | H | H | Me | Me | Et3N*2HF | 2,5 | 1,6 | 4 | 3:1 |
In case of compounds, containing two carb-ethoxy groups at multiple bonds of diene and electron-donating substituents (for example, methyl groups), anodic fluorination produces mixture of hem-difluoro-and vicinal difluoroderivatives (ratio 3:1) [102]. This shows the realization of two fluorination processes pathways.
Olah's reagent (HF/Py) is most widely used as electrolyte, and methylene chloride is used as solvent [49,50]. Another path is connected with use of Et3N*3HF as electrolyte , and acetonitrile as solvent [31]. Carbonyl compounds, containing enol form of -CH2- fragment in 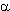 -position are fluorinated with high selectivity into
-position are fluorinated with high selectivity into 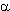 -position, producing as a rule monofluoroderivatives. It is important, that phenyl substituent should be at carbonyl group or this fragment should be in cyclic system (table 11) [44]. This circumstance results in the fact that anodic fluorination is widely used for selective introduction of fluorine atom into compounds, having electron-seeking groups.
-position, producing as a rule monofluoroderivatives. It is important, that phenyl substituent should be at carbonyl group or this fragment should be in cyclic system (table 11) [44]. This circumstance results in the fact that anodic fluorination is widely used for selective introduction of fluorine atom into compounds, having electron-seeking groups.
to be continued
Fluorine Notes, 2002, 25, 1-2
