Fluorine Notes, 2002, 24, 1-2
Fluorine notes, Vol. 5(24) 2002
The electrolytic method of fluorination in the medium containing the complexes of anhydrous hydrogen fluoride and trialkylamines.
G.G.Furin
Novosibirsk Institute of organic chemistry named after N.N.Vorozhtsov
Siberian branch of the Academy of Science of Russian Federataion
Fax: +7-3822-344752
e-mail: furin@nioch.nsc.ru
ABSTRACT
The review generalizes and systematizes the latest date on fluorination of the various classes of
organic compounds by the electrolytic anodic oxidation method in the medium of electrolyte –
the complexes of anhydrous hydrogen fluoride and trialkylamines. An analysis was performed of
the basic achievements of the method of one atom fluorine introduction. The facts having an influence
on the process and influence of electron-seeking substitutes arranged by CH-fragment and by heteroatoms
of VIA group are shown. The examples are given of the useful applications of this method for
the synthesis of aromatic and heterocyclic compounds as well as compounds having XCHF fragments,
where X=S, Se, Te, as the potential intermediate products for synthesis of the preparations possessing
a biological activity. The new efficient production methods of monofluorine – containing organic
compounds and their role in organic synthesis are discussed.
Contents
Introduction
-
Electrolytic fluorination of organic compounds in the presence of the
electrolyte containing the complexes of anhydrous hydrogen fluoride and
trialkylamines.
- 1. Anodic monofluorination of aromatic compounds.
- 2. Electrolytic fluorination of heterocyclic compounds.
- 3. Anodic fluorination of the organic compound with CH-fragment containing electron-seeking
substitutes.
- 1. Anodic monofluorination of aromatic compounds.
-
Behavior of organic derivatives of the VI group elements, having CH-fragment in alpha position
to heteroatom in the electrolytic solutions containing hydrogen fluoride.
Conclusions
References
INTRODUCTION
As applied to the synthesis of fluororganic products hydrogen fluoride and fluorine are of great importance and their reactions with organic compounds are the most direct and economical method producing these compounds. Electrochemical fluorination in hydrogen fluoride solution at the nickel electrodes enables to perform the synthesis of perfluorinated compounds with complex structure. The compounds are hardly can be produced by known procedure which intensifies the interest to this fluorine introduction method and a number of its examples is continuously increasing (1-3), while the significance of the process is growing. This method finds a wide application to produce perfluorinated compounds (1).
The version of the electrochemical fluorination method – an electrolytical fluorination method at the platinum electrodes in the electrolytes containing conducting complexes of hydrigen fluoride with tertiary amines leads to monofluoroderivative formations. A mild reaction conditions as well as simplicity and safety of operations with fluorination system and regioselectivity of the process make this process perspective especially to synthesis of difficult compounds with one or two fluorine atoms (4-6). The reviews concerning this method are in the works (7-15).
Electrochemical system Anode-Electrolyte-Cathode differs from an orginary reduction-oxidation system in spatial interface of oxidation-anodic and reduction cathodic parts of a reduction-oxidation reaction. When an external DC source used, this system permits a needed value oxidation potential at the anode and of reduction potential at the cathode to be continuously adjusted and to be precisely maintained within stability of this electrolytic system.
Both of these fluorination methods complement each other. This effers strong possibilities to produce perfluorinated organic compounds as well as mono- and difluoro- organic molecules. In both cases hydrogen fluoride is a solvent and a fluorination reagent. And, fluoride-ion is of primary importance in the cases.
1.Electrolytic fluorination
of organic compounds in the presence of the electrolyte containing the
complexes of anhydrous hydrogen fluoride and
trialkylamines.
For electrolytic fluorination method the new nonviscous at room temperature electrolytes of
R4NF* nHF or R4NF *3HF was used. The electric conductivity of such
solutions reported in Table 1.
Table1. The electric conductivity of the solutions in MeCN containing 1 mol/dm3R4NF*nHF и Et3N *3HF(1b)
|
Electrolyte |
Electric conductivity 10-1 S/cm |
| Me4NF*2HF | 59,6 |
| Et4NF*2HF | 72,9 |
| n-Pr4NF*2,2HF | 65,8 |
| n-Bu4NF*2,2HF | 48,1 |
| Et3N*3HF | 36,3 |
| Me4NF*2,8HF | 59,2 |
| n-Pr4NF*3HF | 67,2 |
| n-Bu4NF* | 50,8 |
| Me4NF*4HF | 116,4 |
| Et4NF*4HF | 89,8 |
Using of the complexes of hydrogen fluoride with the bases as electrolytes brings to selective introduction of one fluorine atom (17-25). Platinum is still a frequently usable electrode but using the anodes made of the other material (for example graphite, rhenium) gives good results. Acetonitrile is the best accessible solvent to perform the process of the electrolytic fluorination, however, the possibilities to use the other solvents, for instance dimethoxyethane, were shown. A high selectivity and a high yield make this fluorination method not only important to produce fluoroorganic compounds but largely perspective to produce the fluorine-containing heterocyclic and the natural substances as well. In spite of a curtain progress on obtaining desired low-fluorinated compounds by this way for now, the method is economically unsuitable due to extraction complexity of the reaction products and low selectivity.
The process of organic compound fluorination including fluorine addition to a multiple bond and hydrogen substitution for the fluorine in a hydrocarbon chain is a reduction – oxidation process due to fluorine possesses the greatest possible electron affinity (4.15 eV) and the highest standard potential of reduction-oxidation reaction (2.87 V).
Compounds with multiple bond undergo the anodic fluorination at a platinum anode in Et3N*3HF/MeCN system. So, the anodic fluorination trans- and cis- 1,2-diphenylethylene brings to the formation of product mixture (2b).
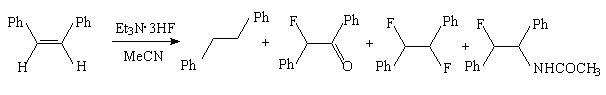
| cis- | 15% | 14% | 14% | 24% |
| trans- | 20% | 5% | 34% |
The anodic fluorination of 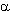 ,
,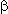 -unsaturated
carbonyl compounds having alkylthioxy group attached to a multiple bond 1(27) and
vinylthioethers 2 (28) in Et3N*3HF electrolyte in the acetonitrile medium
results in the formation of
-unsaturated
carbonyl compounds having alkylthioxy group attached to a multiple bond 1(27) and
vinylthioethers 2 (28) in Et3N*3HF electrolyte in the acetonitrile medium
results in the formation of 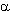 -fluoro-
-fluoro-
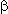 -thio-
-thio-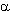 ,
,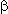 -unsaturated
carbonyl compounds and
-unsaturated
carbonyl compounds and 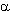 -fluorodialcylsulfides.
-fluorodialcylsulfides.
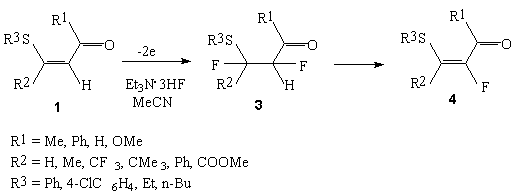
Vicinal difluoroderivatives 3 are originally formed, then the substances are dehydrofluorinated by E1cB mechanism, giving the monoflorination product 4 with the high selectivity. The Data of the anodic fluorination of vinylethers reported in Table 2.
Table 2. Anodic fluorination of vinylethers (28).
|
Substrate |
EV |
F/mol |
Yield of reaction products,% |
||
|
R1 |
R2 |
5 |
6 |
||
| H | H | 1,0 | 3,2 | 8 | - |
| Ph | H | 1,0 | 2,5 | 72 | - |
| Ph | Ph | 0,9 | 2,2 | 35 | 35 |
| Ph | Me | 1,0 | 2,5 | 37 | 30 |
| Ph | COOMe | 1,2 | 3,5 | 75 | - |
| COOMe | H | 1,4 | 2,3 | 78 | - |
| COOMe | H | 1,4 | 2,9 | 29 | - |
It is interesting that trans-2 (phenylthio) styrene (mixture E/Z=90/10) gives  ,
,
 ,
,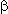 -trifluorosubstituted sulfide 7 (28).
-trifluorosubstituted sulfide 7 (28).
1.1. Anodic monofluorination of aromatic compounds
Knuniants and Rozhkov [6, 22, 24] have discovered the possibility of a partial fluorination of aromatic
compounds on a platinum anode at potentials below the standard potential of fluorine emission, the
reaction being carried out in an electrolytic medium comprising acetonitrile and bifluorides of quaternary
ammonium bases via generation of the carbocation of an aromatic substrate upon its oxidation on the
anode followed by reaction with fluoride ion. This approach provides a basis for many processes yielding
monofluorinated derivatives of the aromatic and heterocyclic series.
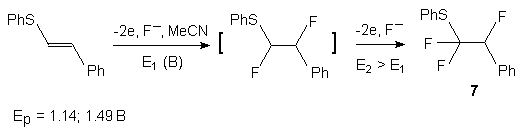
The process of selective anodic fluorination runs according to the ECEC mechanism (electrochemical reaction -> chemical reaction-> electrochemical reaction-> chemical reaction). The first stage is generation of the cation-radical of a substrate by its electrochemical oxidation, the cation-radical reacting with fluoride-ion yielding a benzenium radical. The subsequent electrochemical oxidation yields a bensenium cation, its stabilization by proton detachment resulting in the final product of the reaction [5].
Upon electrochemical fluorination on a platinum anode at 2.5 V (relative to Ag/Ag+ at 0.01 M] in acetonitrile containing R4NF * mHF (where R = Me, Et, I-Pt, n-Bu; n = 2,3,4) and Et3N* 3HF, benzene forms fluorobenzene at a 20-45% yield [29-32]. A substantial yield of fluorobensene (55%) has been obtained by anodic fluorination of benzene in Et4NF*3HF as electrolyte [33, 34]. Monaco et al [16] have shown that, in the Et4NF*3HF system, only fluorobenzene is formed as the principal product contaminated by minor amounts of 1,4-difluorobenzene and products of further fluorination. In the presence of other solvents, tetrafluorobenzene derivatives are obtained [35]. A number of reports describe electrochemical fluorination of benzene in R4NF* mHF yielding fluorobenzene in combination with not only isomeric difluorobenzenes (mainly 1,4-difluorobenzene [29, 36, 37] but, also, fluorinated cyclohexadienes [29]. The final products of fluorination of benzene and fluorobenzene are electrochemically stable 3,3,6-trifluoro-1,4-cyclohexadiene (yield 66-70%) and 3,3,6,6-tetrafluoro-1,4-cyclohezadiene (yield 71-76%).
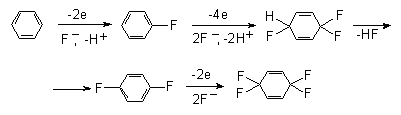
The anode material is usually platinum, however, ruthenium and its alloys with more than 80% Rh may be employed as well, the electrolyte used in such cases being RR1R2R3NF*(HF)n [38].
The accumulation of halogen atoms in benzene ring results in high yields of fluorinated cyclohexadienes. Thus, electrolytic fluorination (platinum anode, Et4NF*mHF) of 1,2,4-trifluorobenzene produces a mixture of 1,3,3,6,6-pentafluorocyclohexadiene-1,4 and 2,5,5,6,6- pentafluorocyclohexadiene-1,3, the total yield being 90% [39]. Fluorinated cyclohexadienes-1,4 will also be formed from 1,4-difluorobenzene and 1,4-difluoro-2-bromobenzene [27].
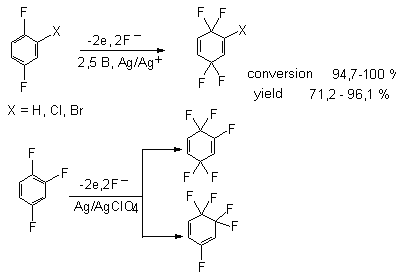
Difluorobenzene and 1,2,3-trifluorobenzene on a Pt anode (2.5 V) in Et4NF *mHF (m = 4,
4.36) and at a 98-99% conversion, as a rule, also yield mixtures of fluorinated cyclohexadienes,
i.e., tetra- and pentafluorocyclohexadienes [40], which is not accompanied by the formation of polymeric
films on the electrode and by discoloration of the electrolyte solution.
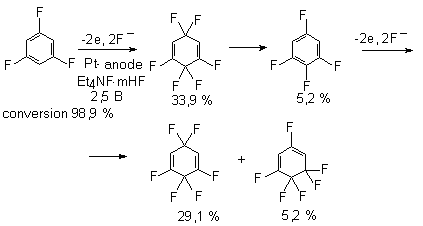
A similar picture is observed upon electrochemical fluorination of bromobenzene [41], chlorobenzene [42, 43], 1-bromo-4-fluorobenzene, and 1,4-difluorobenzene [41].
Anodic fluorination of chlorobenzene in Et4NF* mHF (m = 4, 4.45, 4.7) on a Pt anode at 2.3V or 2.5V results in primary fluorination products 1-chloro-2-fluorobenzene and 1-chloro-4-fluorobenzene at a 1:3 molar ratio [42]. At the final stage of electrolysis, these compounds are fluorinated resulting in a high yield of 1-chloro-3,6,6-trifluoro-1,4-cyclohexadiene or a mixture of 3-chloro-3,6,6-trifluoro1,4-cyclohexadiene, 2-chloro-5,6,6-trifluoro-1,3-cyclohexadiene, and 3,3,6,6-tetrafluoro-1,4-cyclohexadiene, respectively.
It should be noted that in the fluorination process it is possible to replace bromine with fluorine. In the first case, 1-bromo-3,6,6-trifluoro-1,4-cyclohexadiene and 3-bromo-3,6,6-trifluoro-1,4-cyclohexadiene have been obtained. Electrochemical fluorination of 1-bromo-4-fluorobenzene in Et4NF*mHF (m = 4,0, 4.45, 4.7) produces 1-bromo-3,3,6,6-tetrafluoro-1,4-hexadiene, whereas the same with 1,4-difluorobenzene results in 3,3,6,6-tetrafluoro-1,4-cyclohexadiene [41]. Besides, replacement of bromine by fluorine is possible under this conditions [41]. The further stages run according to fluorination of 1,4-difluorobenzene.
Thus, the use of tetraalkylammonium fluoropoly(hydrogen fluoride), R4NF*mHF (R = Me, Et, Pr, m> 3.5), as an electrolyte for electrochemical fluorination of aromatic compounds containing several fluorine atoms usually yields fluorinated derivatives of cyclohexadienes and cyclohexene [43, 44].
Anodic oxidation of phenol in Et3N* nHF results in 4,4-difluorocyclohexa-2,5-diene-1-on, and subsequent reduction with zinc in an acid aqueous medium provides for the possibility to obtain para-fluorophenol at a 60% yield [36, 37]. This approach may become an alternative to oxidative methods for the synthesis of para-fluorophenol.
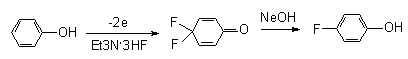
It should be noted that anodic oxidation of anisole and phenetole, which have fluorine atom in the para-position, results in the formation of fluorinated dienons, which is possibly caused by dealkylating of an intermediate radical [45].
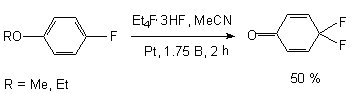
Electrochemical fluorination of (trifluoromethyl)benzene on a platinum anode at 2.5-2.7 V (relative to Ag/Ag+, 0.01 M) in Me4NF*mHF or Et4NF*mHF (m > 3.5) produces 2-fluoro-1-trifluoromethylbenzene and 3-fluoro-1-trifluoromethylbenzene at similar yields [45]. However, these products undergo further fluorination producing fluorinated cyclohexadienes. Thus, a further electrolysis converts the first compound into 3,6,6-trifluoro-1-trifluoromethyl-1,4-cyclohezadiene, and the second one into a mixture of 5,6,6-trifluoro-1-trifluoromethyl-1,3-cyclohexadiene, 3,6,6-trifluoro-1-trifluoromethyl-1,4-cyclohexadiene, and 5,5,6-trifluoro-1-trifluoromethyl-1,3-cyclohexadiene [46].
During electrolysis of isomeric fluorotoluenes in Et4NF *4HF, fluorination of the benzol ring and methyl group occurs [47]. Two pathways realized as the generic case are shown on the scheme below:
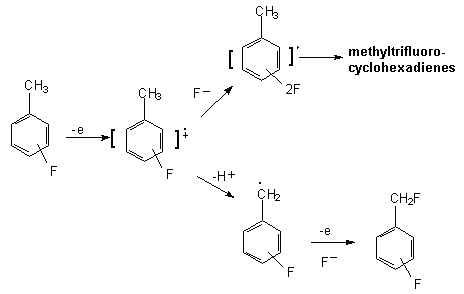
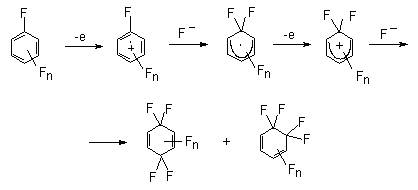
In the general case if benzene ring contains CH2E group process proceeds on the following way. At first the cation-radical is generated and then either it reacts with fluoride ion or proton elimination occurs. In the second case, benzyl-radical by its electrochemical oxidation yields benzyl-cation. The later reacts with fluoride-ion forming side chain fluorination product.
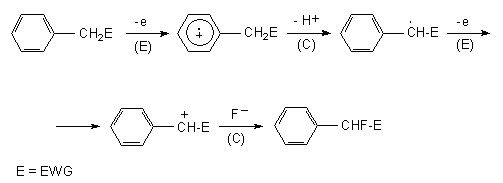
The anodic oxidation of polymethyl- and polyethyl derivatives of benzene in chloromethylene containing Et4NF or Et3N*3HF (44) was reported. The anodic fluorination of polymethyl benzenes in acetonitrile solution results in fluoromethylderivatives (Table 3) (49). The electrode material is platinum. The yield of monofluoromethylderivatives depends on stabilization of the intermediate cation by substitutes or on electrolyte concentration. Thus in case of hexamethylbenzene monofluoroderivatives amount to 88% at the electrolyte concentration is 0,1 mol, and 92% at 0,3 mol. At the same time acetonitrile addition leads to the formation of ArCH2F 8 and ArCH2NHCOCH3 9 mixture. Propotion of products in the mixture depends on the number of methyl group in benzene ring (26).
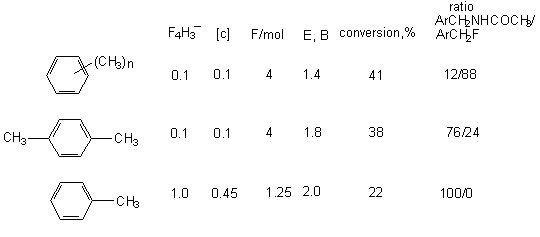
It is supposed that the process runs according to the scheme, which proposes the generation of intermediate
benzyl-cation from in aromatic substrate (50).
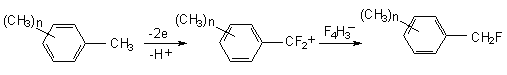
Table 3. Elecrtrolytic fluorination of polymethylbenzene (platinum electrodes, 0,1 M Et4NF•3HF electrolyte, fluorinate reagent: substrate ratio 4:1), MeCN (49).
|
Substrate |
Potential, V |
Electrolyte concentration, mol |
Monofluoroderivatives |
|
|
8 |
9 |
|||
| Tolyene | 1,9 | 0 | ||
| 1,4 –dimethylbenzene | 1,8 | 25 | 9 | |
| 1,2,4-trimethylbenzene | 1,7 | 30 | 8,5 | |
| 1,2,4,5-tetramethylbenzene | 1,4 | 0,1 0,3 |
69 78 |
43 52 |
| pentamethylbenzene | 1,4 | 81 | 53 | |
| hexamethylbenzene | 1,4 | 0,1 0,3 |
88 92 |
51 77 |
Mono- and difluoroderivatives of polycyclic aromatic compounds (perylene, pyrene, triphenylene (Bu4NF*3HF
electrolyte) (51, 52), anthracene, 9-methylanthracene, 9-phenylanthracene (Et3N*3HF electrolyte)
(52, 53) are produced by electrolysis of its solutions in anhydrous acetonitrile at the platinum
or graphite electrodes. The authors concluded that the formation of anodic fluorination products
is controlled more likely with Coulomb's factor (charge of organic electrophile) than thermodynamic
factor (proximity of energy MO HxFx+1- and ArH) (52).
Anodic
fluorination of 3-alkylidene in the medium Et3N3HF/MeCN at the platinum electrodes brings
to the fluoroacetamide formation (54).
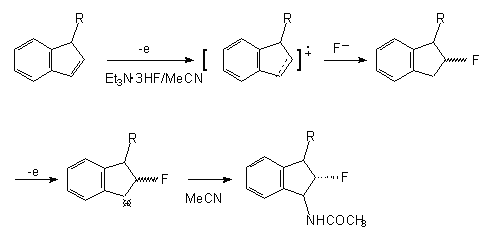
In the case of stilbene and the compounds with multiple bond, an electrolysis in Et4NF nHF
and Et3N•3HF electrolytes mainly gives the products of fluorine cis-addition to multiple
bond (44). That was shown for cyclohexene and indene in much the same way (55).
To be continued
Fluorine Notes, 2002, 24, 1-2
