Fluorine Notes, 2002, 24, 5-6
Fluorine notes, Vol. 5(24) 2002
Study on regularities in electrochemical fluorination of toluene and benzotrifluoride in the presence of triallylamine.
V.A. Matalin, G.I.Kaurova
Federal State Unitary Enterprise Russian Scientific Center "Applied chemistry",
197198, St.Petersburg, Dobrolubov ave. 14.
Electrochemical fluorination (ECF) in anhydrous hydrogen fluoride on nickel electrodes is a convenient
and frequently used method to convert non-fluorinated or partly-fluorinated organic substances to
their perfluorinated analogues, and what is the most important is that the resulting molecule reserve
both the functional groups and the structure of the precursor with heteroatom in it if available.
The essence of the electrochemical fluorination method is that direct current under low voltage is
passed through a dilute solution or through a suspension of the original substance in anhydrous hydrogen
fluoride. Special additives may be applied, if necessary, to impart conductivity to the solution
or to the suspension. J. H. Simons, a well-known American researcher, was the first to give the description
of the method in 1940th [1].
The usage of ECF, though bearing some similarities with, has a number
of advantages over fluorination with elemental fluorine or cobalt trifluoride, both replacing hydrogens
for fluorines and leading to fragmentation of the molecular carbon skeleton [2]. The main advantages
are as follows:
- Hydrogen fluoride itself is applied for the source of fluorine, thus excluding the stage of elemental
fluorine or cobalt trifluoride preparation.
- Exhausting (full) fluorination is carried out in
a single apparatus (that is in an electrolyzer), to result in isolation of the appropriate perfluorinated
substance.
- In spite of the destruction of the molecular carbon skeleton that always has
place along with regrouping that occurs sometimes, the method permits to reserve in great part the
functional groups available in the initial substance.
Among so many perfluorinated substances manufactured through the ECF method, it is amines, ethers, esters and some derivatives of fluoroanhydrates of carbonic acids or sulfonic acids that are of most interest for industry. Great interest is now being demonstrated in fluorine-containing sulfur-containing organic substances considered as perspective fluorinating agents, raw material for electrolytes to be used in chemical current sources, or feedstock in the synthesis of novel organofluoric substances. Perfluorocycloparaffins usually are not included in the list because all efforts to prepare them through ECF were unsuccessful as a rule. For instance, both K. Nyberg [3] and the Japanese team [4] found the yield of perfluoromethylcyclohexane in electrochemical fluorination of toluene to be very low (1).
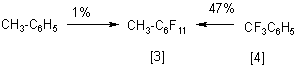
This low yield of the target product, considerable gumming of the initial substance, poor solubility
of the initial substances in anhydrous hydrogen fluoride, and, consequently, low electric conductivity
of electrolyte, and the need in the usage of electrolytic additives √ all this led to abandonment
of the efforts to produce those substances through the method of ECF [2].
However, the
development of technology for the manufacture of perfluorinated cyclic paraffins is still an important
practical issue because those fluorocarbons possess extraordinary combination of their physico-chemical
properties. They have relatively low boiling temperatures that are not consistent with their molecular
weights; they also have low critical temperatures and pressures, high liquid densities, high expanding
coefficients, while their surface tensions and refraction indices are much lower than those of any
other liquid. The compressibilities of those liquids are higher than those of any others, and that
is why the speed of sound propagation is very small in them. Those substances possess perfect electrical
properties. The common distinctive feature of perfluorolcycloalkanes is their unique physical and
chemical stability. From biochemical point of view fluorocarbons are considered as ⌠practically nontoxic■,
what means their toxic coefficient to be as low, as possible.
In the present work the possibility
both to increase the yield and to improve the parameters of electrochemical fluorination of aromatic
substances was studied in the course of electrochemical synthesis, using as an example fluorination
of toluene and benzotrifluoride in the presence of triallylamine.
Experimental technique.
Electrochemical fluorination was carried out in a carbon steel Simons-model electrolyzer of volume 0,66
l. The electrolyzer was equipped with a reflux condenser intended for condensation of hydrogen fluoride
carried by electrolysis gas and for its recycling into electrolyte. A coiled tube with circulated
cooling water in it was provided within the electrolyzer in order to cool electrolyte. The electrodes
formed a package that consisted of alternating anode and cathode plates made of nickel. During the
experiments the current load was constant, its value being 15A. The current density was as well constant
in all tests, and its value was 0,03A/cm2. The effective anode area was =504 cm2,
the effective cathode area was the same.
The synthesis was carried out through electrolysis
of the solution that contained 5% by weigh of benzotrifluoride + 5%by weigh of triallylamine and
the solution that contained 5 % tolueneЮ + 5% by weigh of triallylamine in liquid anhydrous hydrogen
fluoride. The electrolysis process was conducted either with periodical dosing (once or twice hourly)
of the initial organic blend or till the voltage reached the value of 6,5 V.
The density of crude
perfluoromethylcyclohexane being considerably higher than that of the electrolyte, the substance
was being accumulated at the bottom of the electrolyzer from where it was poured out at regular intervals.
The crude product was poured under water and neutralized with soda. The crude product was then treated
with 15% water-alcohol alkaline solution in order to remove the residual non-fluorinated impurities
and dried with the help of silica gel.
The dried crude product, now free from hydrogen
fluoride and non-fluorinated impurities, was then directed to rectification where the target product
of purity 98.0-99.5% was isolated. The product was analyzed using the method of gas-liquid chromatography
(GLC). The GLC analysis was carried out with the help of ⌠Tzvet-100■ chromatograph (made in Russia)
equipped with a katharometer and a column packed with silochrome-80 containing 20% ![]() ,
,![]() ,
,![]() √tris-
√tris-![]() -cyanacetophenone.
-cyanacetophenone.
The experimental results for the ECF of toluene
and benzotrifluoride in the presence of triallylamine are shown in Tables N 1 and 2.
It should
be noted that in the course of the experiments, at each stage (200 A.h/l), the analysis was made
as follows: electrolyte was analyzed for the content of hydrogen fluoride and triallylamine, gas
phase was analyzed with the help of chromatography, crude product was discharged and analyzed. Anode
gas always contained the impurities as follows: CF4, C2F6, NF3,
F2O, and a number of other break-down products of the fluorination process. Hydrogen was
the product of the cathode reaction.
Experimental results and discussion
The expensiveness of perfluorinated substances is the main obstacle to their more wide popularity and
implementation in industry contrary to their unique properties. High prime costs of perfluorinated
products is due to:
1. Low yields of the target products in the synthesis if starting from their
hydrocarbon analogues.
2. Negative effect of electrolytic additives (NaF, etc.) both on
the yield of perfluorinated products and the longevity of the electrolysis process.
3. Small
content (30-50%) of the target perfluorinated products in the crude product due to high share of
destructive fluorination in the process.
4. Strong gumming of electrolyte that also has
influence on the longevity of electrolysis.
It is well known [3] that many cyclic
hydrocarbons when dissolved in liquid anhydrous hydrogen fluoride do not conduct electric current.
In such cases some electrolytic additives have to be added to the electrolyte, either inorganic or
organic substances are applicable for such additives [6], [7] and [8]. The usage of inorganic fluorides,
e.g., sodium fluoride, for such an additive results in the considerable increase in anode corrosion.
The slum formation leads to decrease in the electrolyte lifetime and does not stop the electrolyte
gumming, and that is why the process becomes unprofitable for industrial applications [9].
In
order to prevent gumming of the initial materials it was proposed to apply for such electrolytic
additives some organic substances containing divalent sulfur, e.g. n-butylmercaptan, dialkylmonosulfide
or dialkyldisulfide [8]. However, environmental regulations rule out the usage of the said substances
in industrial applications due to the pungent objectionable odor of mercaptan. It also should be
underlined that the said sulfur-containing substances easily undergo electrochemical fluorination,
and considerable amount of hydrolytically non-stable perfluorinated substances are formed that contain
six-valent sulfur. As those substances have got but only limited demand in industry the remaining
material after separation of the target product stays unclaimed thus making the full-scale process
even more expensive and les profitable.
In our earlier work [5] it has been shown that
simultaneous fluorination of a number of organic substances attributed to different classes of organics
makes it possible to increase the content of the target products in the crude product and to diminish
destructive fluorination.
Besides of that, one may increase both the yield of the target
product and the productivity of the electrolyzer if partly fluorinated organic substances are used
for initial raw material.
But partly fluorinated organic substances are often insoluble
and do not conduct electric current in hydrogen fluoride.
In our present work we have studied
the process for preparation of perfluoromethylcyclohexane through ECF of benzotrifluoride and toluene
with addition of triallylamine to each of them.
From the data of Tables 1 and 2 one may see that
the best process parameters were obtained
when benzotrifluoride had been applied for the
initial substance. E.g., the yield-by-current for the target product was 78%, and the yield-by-substance
was 77% in case of benzotrifluoride, those being correspondingly 20% and 5% in case of toluene. It
should be also taken into account that electrolysis of toluene proceeds for rather short time-period
only, and after that the voltage goes up abruptly and strong gumming of the initial substance has
place.
The current density was chosen to be 0,03 A/cm2. It should be noted that
the current density
was high enough, because it is commonly known from published data that
similar studies had been carried out under considerably less load, namely under 0,001-0,002 A/cm2.
The authors probably had in mind to diminish the effect of the above-mentioned destructive fluorination
process. However, when the decrease in the destructive fluorination was inessential (by 5-10%), the
loss in productivity was considerable (1,5 √ 2 times).
From the above one may conclude that the
suggested method allows to improve some indices of the process for electrochemical synthesis of perfluoromethylcyclohexane
from benzotrifluoride and to decrease its prime cost due to simultaneous production of some other
reaction products of commercial interest, namely perfluorinated tertiary amines.
It should be
as well underlined as follows. Perfluoromethylcyclohexane was being manufactured by ⌠ICI Chemicals■
who produced it through fluorination on a catalyst [10]. The new method for the substance preparation
through electrochemical fluorination in liquid anhydrous hydrogen fluoride may possibly be remarkable
for operating of less complicated equipment, higher yield and lower prime cost of the target product.
As it has already been mentioned earlier, the target product contains up to 20% of the carbon skeleton
isomerisation products, and those form a kind of eutectic with the main product (perfluoromethylcyclohexane)
that decrease the freezing temperature to minus 65oC, while the freezing temperature of
pure perfluoromethylcyclohexane is minus 35oC.
The suggested mechanism of the
reactions is as follows:
| main cathode process | |
| main anode process |
Electrode reaction in summary:
![]()
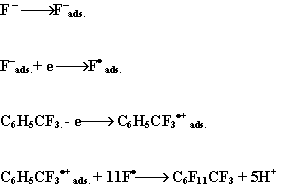
It is probable that the reaction mechanism was the
same in the case of ECF of toluene at the very first moment of the reaction. However, oxidation dimerization,
anode condensation and polymerization of intermediates begin prevailing with the passage of time.
Conclusions
1. Here we have demonstrated the possibility to produce perfluoromethylcyclohexane in the presence of
some novel electrolytic additives.
2. The process may probably be classified as simultaneous
fluorination of substances that belong to two different chemical substance classes.
3. Besides
of perfluoromethylcyclohexane, the second main electrolysis product is perfluorinated tertiary amine.
Perfluorinated tertiary amines are widely applied in industry for dielectric liquids.
4. Perfluorolcyclohexane
is formed as the reaction by-product due to decomposition. Perfluorolcyclohexane may also find applications
in many industrial sectors.
The studies in the field are in process.
Table 1
Process parameters for ECF of benzotrifluoride and toluene in the presence of triallylamine.
| Electrolysis parameters | Benzotrifluoride | Toluene |
| Additive of initial substance, g |
127,4 | 62,5 |
| Additive of triallylamine, g | 121,9 | 75 |
| Total organic additives | 249,3 | 137,5 |
| Manufactured crude product, g | 414 | 49,5 |
| Electrolysis time, hours |
52,5 | 32,5 |
| Current density A/cm2 |
0,03 | 0,03 |
| Amount, A.h |
787,5 | 440 |
| Amount, A.h/l |
1193 | 666,5 |
| Crude product, yield-by-current, % | 78 | 20 |
Table 2.
Per-cent content of main components of crude product in synthesis of benzotrifluoride and toluene in the presence of triallylamine.
| Initial substance | Main components of crude product, % by weigh | ||
| Perfluoromethylcyclohexane | Perfluorolcyclohexane | Perfluorotripropylamine and perfluorocycles | |
| Benzotrifluoride | 57 | 10 | 20 |
| Toluene | 10 | 5 | 65 |
References
- J. H. Simons Pat. US 2519983; E. A. Kauck and J. H. Simons Pat.US 2616927, Pat.US 2613151.
- Abe T. Nagase S. Chapter 1. Electrochemical fluorination (Simons process) as a route to perfluorinated organic compounds of industrial interest. Ed. R. E. Banks 19-43, (1982).
- K. Nyberg, Acta Chem. Scand., 24, 1609 (1970); 25, 534, 2499, 2983 (1971); 27, 503 (1973).
- M. Yonekura, S. Nagase, H. Baba, K. Kodaira, and T. Abe, Bull.Chem. Soc. Jpn., 49, 1113 (1976).
- Patent appl. RU 2002118418, 08.07.2002
- Ftor i ego soedineniya. Pod redaktsiej j. Simonsa. Izdatel'stvo inostrannoj literatury. Moskva, 1953,S.; M. Beizer, H. Lund.
- Organicheskaya ehlektrokhimiya, t. 2, s. 781-788, M., Kkimiya, 1988.
- R.D. Danielson and J.W. Sargent, Pat. USA 3028321, C25B 3/08
- The Role of adsorption in electrochemical perfluorination of alkylsulfofluorides. G. Cauquis, B.Keita and G.Pierre. J. Electroanal. Chem., 100 (1979) 205 √ 215.3).
- Slinn D.S.L., Green S.W. Chapter 2. Fluorocarbon Fluids for use in the electronic industry.45-81, (1982).
Fluorine Notes, 2002, 24, 5-6
