Fluorine Notes, 2002, 22, 1-2
Use of hydrogen fluoride and its complexes with bases for introduction of fluorine atoms into organic molecules
G.G.Furin
Novosibirsk Institute of organic chemistry named after N.N.Vorozhtsov
Siberian branch of the Academy of Science of Russian Federataion
Fax: +7-3822-344752
e-mail: furin@nioch.nsc.ru
Annotation
The review summarizes and systematizes up-to-date data on fluorinating ability of anhydrous hydrogen fluoride and its complexes with bases of unsaturated organic compounds, alcohols, diazoketones, hydrazones and oximes of ketones, 3,3-dialkyl-1-aryltriazenes, aryldiazosulfides etc.. It contains an analysis of main achievements in use of anhydrous hydrogen fluoride as a fluorinating agent to produce ozone-friendly freons in gas and liquid phases both without catalysts and in the presence of latter. There has been examined factors influencing opening three-membered cycles containing oxygen and nitrogen atoms. The review contains examples of practical application of different groups of fluoroorganic compounds, rational methods of their production and their role in development of modern industry .
Contents
Introduction. Hydrogen fluoride as a basic stock substance in chemical industry.
1. Fluorination with anhydrous hydrogen fluoride and its complexes with bases of compounds from different classes.
1.1.Hydrofluorination of unsaturated compounds
1.1.1. Influence of anhydrous hydrogen fluoride
on unsaturated compounds
1.1.2. Hydrofluorination of unsaturated compounds by hydrogen fluoride
complexes with bases
1.1.3. Reactions of anhydrous hydrogen fluoride and its complexes containing
bases with acetylene derivatives
1.1.4. Fluorination of alkenes with hydrogen fluoride in
the presence of catalysts
1.1.5. Fluorination of unsaturated compounds with hydrogen fluoride
in the presence of electrophilic reagents
2. Processes of replacement of functional groups with fluorine atoms.
2.1. Replacement of oxy-group with fluorine under effect of hydrogen fluoride complexes containing
bases.
2.2. Reactions with hydroxylamines, hydrazones and oximes of ketones.
2.3. Reactions with diazoketones, 3,3-dialkyl-1-aryltriazenes and aryldiazosulfides.
2.4. Exchange reactions of haloids under effect of hydrogen fluoride in the presence of catalysts
3. Opening nitrogen- and oxygen-containing three-membered heterocycles
3.1.Opening an epoxy ring by anhydrous hydrogen fluoride and its complexes with bases.
3.2.
Opening nitrogen-containing three-membered heterocycles.
Conclusion.
References
2.2. Reactions with hydroxylamines, hydrazones and oximes of ketones.
The Bamberger rearrangement of N-arylhydroxylamine or arylazide under the effect of anhydrous hydrogen fluoride results in formation of 4-fluoroaniline [251,252].
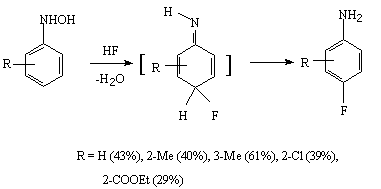
The reduction of nitrobenzene (with bismuth or lead) in a medium of anhydrous hydrogen fluoride runs through an intermediate formation of arylhydroxylamines which convert to fluoroanilines in the hydrogen fluoride medium [253-255].

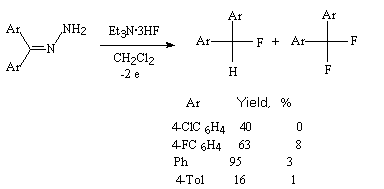
Hydrazones of ketones under the influence of 60% HF/Py or 59% HF/poly-4-vinylpyridine in the presence of N-bromsuccinimide convert to hem-difluoro-derivatives [256]. In case of oximes of ketones the presence of chlorine is necessary, therefore a mixture of products is obtained (hem-dichloro-, chlorofluoro- and difluoro-derivatives) [257].
An electrochemical process (1.3 mA/cm2) can be used for hydrazones of diarylketones that makes possible to obtain a monofluoro-derivative only [258].
2.3. Reactions with diazoketones, hydrazones and oximes of ketones.
Reactions of diazoketones with 70% HF/Py system in the presence of a source of cations of a haloid give appropriate fluoroketones or fluoroparaffines [2]. Diazaalkanes, diazaketones and diazaacetates can be fluorinated at 0oC under the effect of this system in 30-95% yield [2,259].
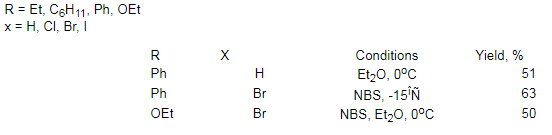
where NBS- bromosuccinamide
The process of 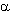 ,
,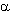 ,-hydrofluorination in the presence of a source of haloid cations, N-bromosuccinamide for example,
in HF/Py system runs according to the halofluorination type (Table19) [2,259].
,-hydrofluorination in the presence of a source of haloid cations, N-bromosuccinamide for example,
in HF/Py system runs according to the halofluorination type (Table19) [2,259]. 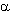 -Fluoro-
-Fluoro- 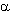 -haloethers,
-haloethers, 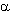 -fluoroketones,
-fluoroketones, 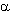 -fluoroethers
are obtained. So, benzyl fluoride was produced in 70% yield according to this method.
-fluoroethers
are obtained. So, benzyl fluoride was produced in 70% yield according to this method.
6 -Bromo- and
6
-Bromo- and
6 -chloro-6
-chloro-6  -fluoropenicilates were obtained from appropriate diazo-compounds and present interesting
products (Table 19). A mixture of endo-3-fluorobornan-2-one (30%) and a product of exo-2-fluorobornan-7-one
(52%) was obtained from norbornane diazo-compound. 2-Diazocholestan-3-one under the effect of
HF/Py system converts to an appropriate fluoride in 50% yield (table 19).
-fluoropenicilates were obtained from appropriate diazo-compounds and present interesting
products (Table 19). A mixture of endo-3-fluorobornan-2-one (30%) and a product of exo-2-fluorobornan-7-one
(52%) was obtained from norbornane diazo-compound. 2-Diazocholestan-3-one under the effect of
HF/Py system converts to an appropriate fluoride in 50% yield (table 19).
Table 19. Reaction of diazo compounds with 70% HF/Py in the presence of N-halosuccinimides.
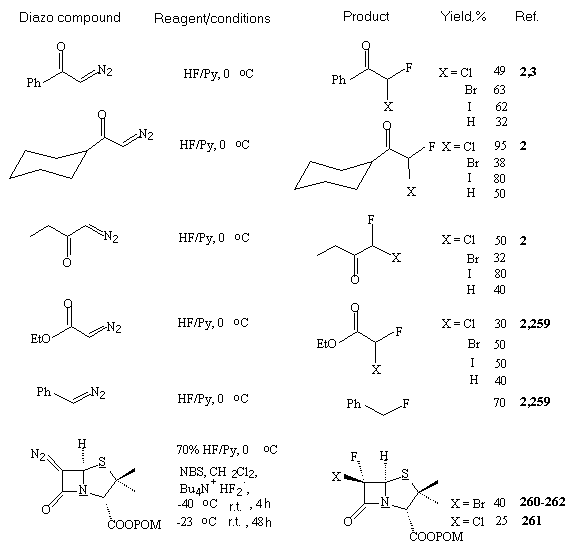
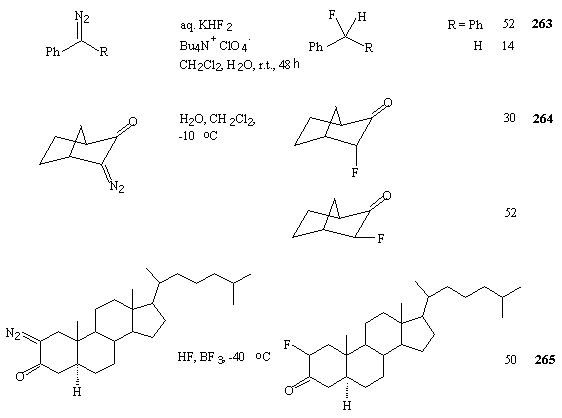
POM = (CH3)3CCOCH2
3,3-Dialkyl-1-aryltriazenes have been known for a long time. They are obtained by condesation of aryldiazonium salt with secondary dialkylamines. It has been found that under the further influence of hydrogen fluoride in an anhydrous medium there was generated aryldiazonium salt which composition runs smoothly to for appropriate fluorobenzenes [266-268].
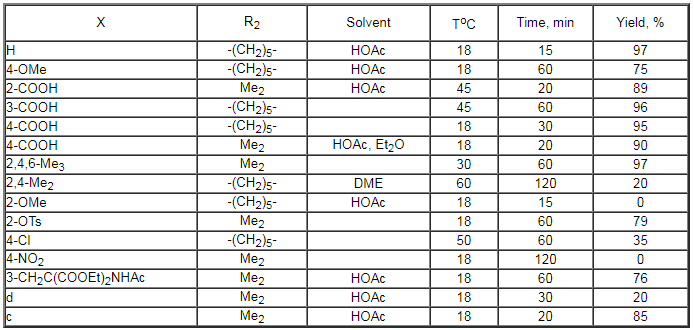
For the first time this approach was proposed by Wallach. But only now it has become an important method to produce fluoroaromatic compounds and fluorine-containing natural compounds. In case of using anhydrous hydrogen fluoride for decomposition of 3,3-dialkyl-1-aryltriazenes the nature of the solvent used is of great importance. So, a mixture of products is obtained in tetrahydrofuran, radical processes (dimerization, reduction) are realised in methene chloride, products of fluorination are obtained in acetic acid (table 20)[269].
Table 20. Conversion of 3,3-dialkyl-1-aryltriazenes to fluoroaromatic compounds under the influence of HF/Py system [269].
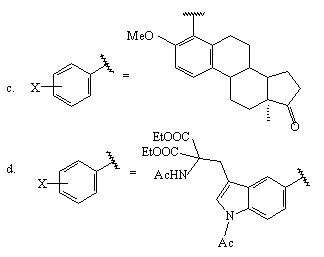
Aromatic and haloaromatic compounds are found the best solvents for this reaction [270]. Against a background of N=N-NH2 group the presence of the OH group in the 4-position of the benzene ring does not impede the fluorination process followed by thermal or photochemical decomposition resulting in formation of 4-fluorophenol.
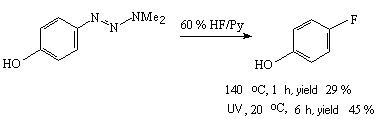
If hydrogen fluoride labeled with 18F isotope is used in the stage of decomposition of intermediate diazo-salt then fluoroaromatic compounds labelled with 18F isotope are obtained, [18F] haloperidol for example. It is particularly important for production of preparations for positron tomography [271].
Also, aqueous hydrogen fluoride can be used for decomposition of aromatic triazenes, here fluoro-derivatives are obtained in a low yield.
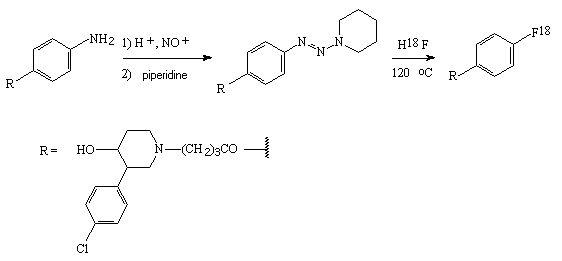
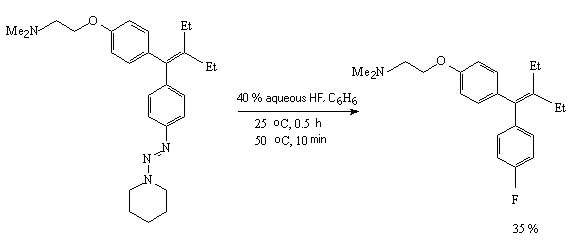
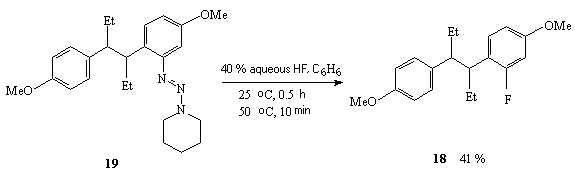
For example, dimethyl ether of 2-fluorohexestrol 18 [270] is obtained under the effect of hydrogen fluoride (anhydrous HF, aqueous HF, HF/Py system) on piperidintriazene 19 in various solvents (tetrahydrofuran, benzene, methene chloride, acetic acid) [270].
An important alternative for fluorobenzene production is decomposition of 3,3-dialkyl-1-aryltriazenes 20 and decomposition of aryldiazosulfides 21 in the presence of silver ions (AgF or AgNO3) which is realised at a stehiometric quantity of the fluoride (table 21) [272]. The Olah reagent is used rather frequently in this method [272].
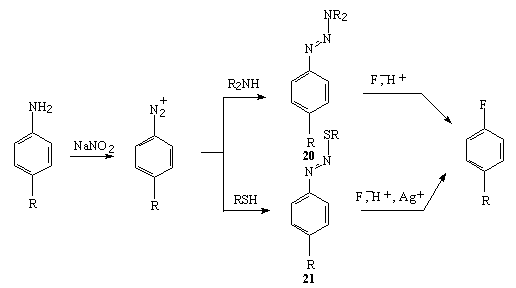
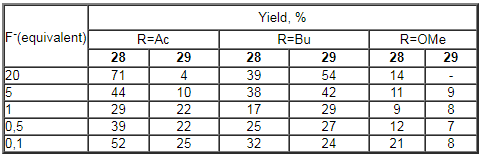
Table 21. Decomposition of aryldiazosulfides to fluorobenzenes promoted by salts of monovalent silver [272].
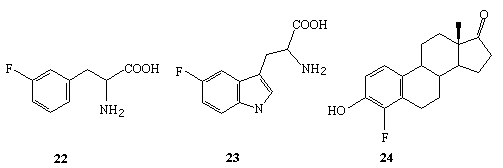
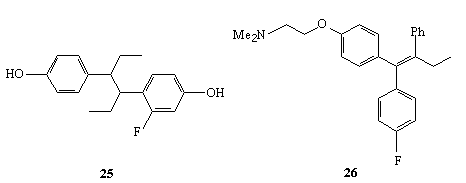
3' -fluorophenylalanine 22 [269], 5' - fluorotryptophan 23 [269],
4-fluoroestron 24 [269], 2' - fluorohexestrol 25 [270] and fluorotamoxyphen 26.The following biologically active compounds can be obtained according to this
way:
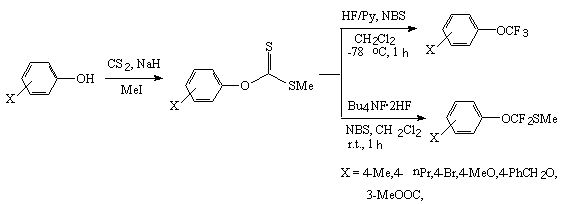
The action of Bu4NF*2HF and HF/Py systems on aryl and alkyl ethers of xanthic acid in CH2Cl2 in the presence of N-bromosuccinimide results in formation of trifluoromethyl ethers [273-275].
In case of ethers of dithiocarbamic acid these systems result in formation of tertiary amines containing one trifluoromethyl group [274].
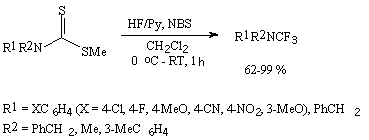
2.4. Exchange reactions of haloids running under the influence of hydrogen fluoride in the presence of catalysts.
An important aspect of synthesis of ozone-safety freons are processes of fluorinaton of halogenated hydrocarbons by means of anhydrous hydrogen fluoride in the presence of the following substances: oxides or fluorides of indium, chromium at 300-500oC [276], chrome-gallium catalysts [277], chromium oxide treated with Ru and Pt [278], chromium precipitated on aluminium oxide [279, 280], zinc, its oxide and fluoride [281], chromium chloride [282]. The process is carried our in gas phase over catalyst.. But catalysts based on chloro-fluorides of antimony were found the most effective [283-286a]. A method to obtain difluoromethane by fluorination of methylene chloride with anhydrous HF in the presence of antimony chlorides runs according to the following scheme:

The reaction rate is described by the equation:
-rCH2CH2 = 2.2*105 exp ( 5.8*107/RT ) * CCH2Cl2 * Ck
-rCH2CH2 = 8.3*106 exp ( 6.2*107/RT ) * CCH2ClF * Ck
Where CCH2Cl2, C CH2ClF, Ck are concentrations of CH2Cl2, CH2ClF and catalysts accordingly.
Processes of production CH2F2from CH2Cl2 by the effect of HF over catalyst are carried out at a temperature of 250oC and above it, the yield is 80% at a contact time >20sec {186a, 286b].
Similarly under effect of HF on chloroform in the presence of fluorides of Cr, Al, In, Bi at 100-150oC a mixture of products CHF2Cl, CHF3, CHFCl2 is obtained , the conversion is above 98% [287]. The following compounds as CF3CH2Cl, CCl4, CH2Cl2,CH3Cl, CCl2F2,C2Cl4 under the influence of anhydrous hydrogen fluoride in the presence of catalyst ( partially fluorinated chromium oxide which is treated with Ru or Pt in amount of 0.61-10 mole%) at 290-380oC and a mole ratio of HF/halogenated hydrocarbon within a range of 1:10 to 10:1 give partially fluorinated hydrocarbons [288]. For example, 1,1,1,2-tetrafluoroethane is obtained, it is used as a refrigerant.
1,1,1,3,3,3-Hexafluoroisopropylfluoromethyl ether, an important inhalation anesthetic, is obtained under the influence of Et2NMe*HF [228a] or ( IPr )2(Et)N HF [288b,c] complexes on 1,1,1,3,3,3-hexafluoroisopropylchloromethyl ether at a temperature of 40-80oC. Similarly other subfluorinated ethers are obtained [288d].
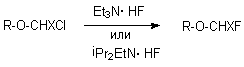
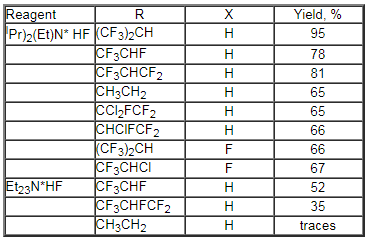
This method was extended for ethane series, for example for synthesis of 1,1-difluoroethane, 1,1,2-trifluoroethane, 1,1,1,2-tetrafluoroethane, pentafluoroethane. They have been found wide application in modern industry as refrigerants, propellents, fire extinguishing agents, reagents for plasmochemical manufacturing super-large integrated circuits, gas dielectrics etc.. Thus, chromomagnesium catalyst was found effective in producing 1,1,1,2-tetrafluoroethane from 1,1,1-trifluoro-2-chloroethane in gas phase [289], 1,1,1,2,2-pentafluoroethane from 1,1,1-trifluoro-2,2-dichloroethane [290], pentafluoroethane from tetrachloroethane [291], 1,1,1,2-tetrafluoroethane and pentafluoroethane from 2-chloro-1,1,1-trifluoroethane [292]. Cr2O3 may be used as a catalyst, the process runs at 300-500oC resulting in hexafluoroethane in high yield and selectivity [293]. SbCl5 acts similarly in liquid phase giving 1,1,1-trifluoroethane from 1-chloro-1,1-difluoroethane [294]. In these cases a role of catalyst reduces to HCl elimination and formation of a derivative of ethylene which is subjected to hydrofluorination under the effect of anhydrous hydrogen fluoride. SnCl4-B(OEt)3 system may be used as a catalyst [295].
If the molecule contains several chlorine atoms then the process may be carried out under conditions of replacement of one chlorine atom only. So, 1,1,1-trichloroethane and HF at 80-100oC and pressure of 10.5-12 kg/cm2 give 1,1-dichloro-1-fluoroethane [296].
It is of interest that in case of fluorine-containing ethanes carrying out the process with HF in the presence of chromium compounds results in an increase of the number of fluorine atoms in the molecule. Thus, interaction of 1,1,1,2-tetrafluoroethane with HF at 302oC gives 1,1,1,2,2-pentafluoroethane. But the yield of the desired product is too low (1.3%)[297].
The method has been also extended for propane and butane series. So, from hexachloropropane under the effect of HF and in the presence of catalyst SbClnF5-n (n=1-5) at 50-100oC there were produced 1-chloro-1,1,3,3,3-pentafluoropropane and 1,1,1,3,3-pentafluoropropane [298]. Similarly when the following substances as halides of molybdenum, tantalum, niobium [299,300], TiCl4 [301], halides of the IV B and V B groups [302,303], SbCl5 [304] were used as catalysts in a reaction with 1,1,1,3,3-pentachloeopropane (freon 240fa) at 100-150oC there was obtained 1,1,1,3,3-pentafluoropropane (freon 245 fa) in a yield above 90%. 1,1,1,2,3,3,3-Heptafluoropropane was obtained in hydrofluorination of 1,1,1,2,3,3,3-heptachloropropane over catalyst [305] and also in hydrofluorination of hexafluoropropane in gas phase over a fluorinated catalyst ( fluorides of aluminium and chromium) [306].
Hydrofluorination of chlorofluorobutanes CClaCF3-aCH2CClbF2-bCH3under the influence of HF results in formation of 1,1,1,3,3-pentafluorobutane [307,308].
The given examples show that the parameters determining the course of hydrofluorination processes are caused by a wider interaction spectrum than interaction of organic substrate with hydrogen fluoride, in particular by processes of generation of unsaturated compounds and their interaction with anhydrous hydrogen fluoride [309-314].
Fluorine Notes, 2002, 22, 1-2
