Fluorine Notes, 2002, 22, 5-6
The study of the process of joint thermal decomposition of chlorodifluoromethane and dichlorofluoromethane.
A.A. Nikolaev, V.G. Barabanov.
It is known that a main method to produce chlorotrifluoroethylene, the very important commercial fluoromonomer, is dechlorination of 1,1,2-trifluorotrichloroethane with zinc [1-3] . But this method has a number of essential disadvantages, the main of them is prohibition of the production of refrigerant 113 as a commercial product according to the Montreal protocol. As a result the cost of raw materials in the process increased considerably.
Chlorotrifluoroethylene, as many other fluorine-containing monomers, can be obtained by means of reactions of thermal decomposition, in particularly by co-pyrolysis of chlorodifluoromethane and dichlorofluoromethane [4,5]. But this process has not been adequately investigated.
The kinetic pattern of the process of thermal decomposition of chlorodifluoromethane are well known, but pyrolysis of dichlorofluoromethane is not studied enough. And so for investigation of the process of joint thermal decomposition of chlorodifluoromethane and dichlorofluoromethane it is necessary to study kinetic behaviour of the reaction of thermal decomposition of the latter.
As since the main stage of this reaction runs according to the first –order reaction, the reverse and side reactions have the second order, the initial alkane was diluted with inert gas (helium) in a ratio of 1:100. Dilution made possible to neglect a volume change during the course of the reaction .
The analysis was carried out within a temperature range of 853-913K, the residence time of the reagents in the reaction zone was 0.08-0.67sec. The main reaction product was dichlorodifluoroethylene. The total concentration of by-products did no exceed 1% and they were not taken into account in calculation of kinetic parameters. Conversion was determined according to a decrease of the initial alkane. The experimental data processing was made by the least squares method according to the equation:
- ln(1-x) = k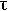 ,
,
where
õ =degree of the initial product conversion
k = reaction rate constant, sec –1
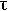 = retention time of the reaction mixture in the reaction zone
= retention time of the reaction mixture in the reaction zone
Fig.1 shows the plot of the reaction of thermal dehydrochlorination of dichlorofluoromethane
in (-ln (1 – x), 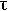 ) co-ordinates.
) co-ordinates.
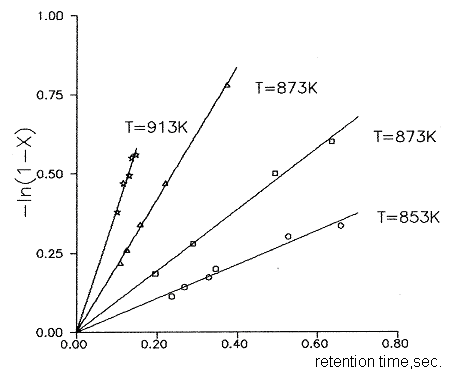
Fig.1 Kinetic dependence of the reaction of thermal dechlorination of dichlorofluoromethane at various temperature values.
The Arrenius parameters of the reaction constants were calculated by a method of least squares according to the equation:
lnk = lnA – Eàêò/RT, where
k = reaction rate constant, sec-1;
Å = reaction activation energy, kJ/mole
Ò = reaction
temperature, K
R = universal gas constant, kJ/mole K
À = exponential factor, sec-1
Arrenius expression for this reaction is as follows:
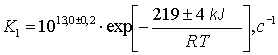
The plot of this dependence in (lnk, 1/T) co-ordinates is given in Fig.2
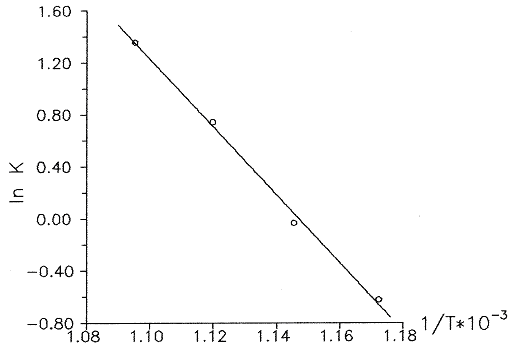
Fig.2 Arrenius dependence of the reaction constant of thermal dehydrochlorination of dichlorofluoromethane
The process of joint thermal decomposition of chlorodifluoromethane and dichlorodifluoromethane was carried out in a continuous reactor in the presence of superheated water vapor.
The following compounds were identified in the pyrolyzate:
C2F3Cl, C2F4, C2F2Cl2, C2F3Cl3, C2F2Cl4, C2F4Cl2 , C2F5Cl, C3F5Cl, C3F4Cl2, C3F3Cl3, C3F6Cl2 , C4F7Cl, C4F6Cl2, ÑÎ.
The main products in this process within a temperature range of 973-1123K, residence time of 0.02-0.12 sec and a ratio of the initial refrigerants of 1:3-0.3:1 were chlorotrifluoroethylene, tetrafluoroethylene and dichlorodifluoroethylene.
These compounds are a product of recombination of difluorocarbenes and chlorofluorocarbenes. A number of papers shows that the pyrolysis of chlorodifluoromethane proceeds according to a carbene mechanism by the reaction [7-10]:
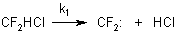
The reaction is reverseible:
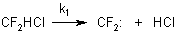
Dichlorofluoromethane, on elimination of hydrogen chloride, generates chlorofluorocarbenes according to the reaction:
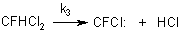
Chlorotrifluoroethylene is formed in recombination of difluorocarbene and chlorofluorocarbene. The recombination of two difluorocarbenes results in formation of tetrafluoroethylene. Dichlorodifluoroethylene is formed in the recombination of two chlorofluorocarbenes
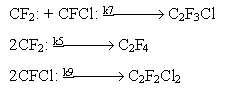
A number of by-products is formed in the process of joint thermal decomposition of chlorodifluoromethane and dichlorofluoromethane, their concentration in the pyrolyzate is considerably lower than that of the three main monomers described above.
As it was convincingly shown by the authors of papers [11,12], hexafluoropropene generating in the process is a product of interaction of difluorocarbene and trifluoromethylfluorocarbene:
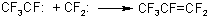 (13)
(13)
Trifluoromethylfluorocarbene is a product of 1,2-migration of fluorine atom in the molecule of tetrafluoroethylene
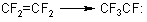 (11)
(11)
Dichlorotetrafluoropropane and chloropentafluoropropene are formed in considerable amounts in the process of co-pyrolysis. A proposed mechanism of formation of these compounds is addition of difluorocarbene and chlorofluorocarbene to the double bounds of tetrafluoroethylene and chlorotrifluoroethylene according the reactions:
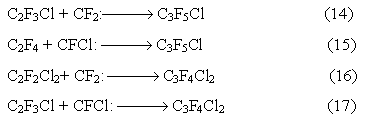
Thus it may be concluded that the reaction of formation of dichlorotetrafluoropropene and chloropentafluoropropene are competitive and draw back carbenes from the reaction of formation of the main monomers ( chlorotrifluoroethylene, tetrafluoroethylene, dichlorodifluoroethylene)
The process of 1,1,2,2-tetrafluorochloroethane formation is well studied and runs by the reaction:
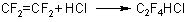 (19)
(19)
Carbon oxide is formed in the course of interaction of water vapor with difluorocarbene and chlorofluorocarbene
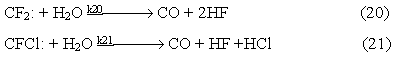
In the process of thermal decomposition of chlorodifluoromethane and dichlorofluoromethane there were found compounds which formation may be explained by radical reactions taking place in the system.
Homolytic splitting C-Hal bonds in chlorotrifluoroethylene , chlorodifluoroethylene may occur at a pyrolysis temperature:
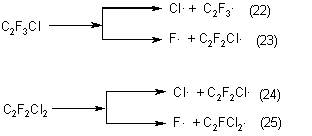
The formation of dichlorotetrafluoroethane and chloropentafluoroethane may be explained by addition of halogen-radical to the multiple bond of the chlorotrifluoroethylene molecule:
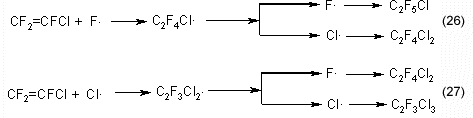
Radicals C2F4Cl. and C2F3Cl2. generating as a result of reactions 26, 27 may interact with a molecule of chlorotrifluoroethylene to form dichlorohexafluorobutene and chloroheptafluorobutene:
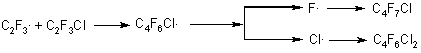
Generation of C2F3., C2F2Cl. radicals may take place with participation of C4F6Cl.radical which can eliminate fluorine and chlorine atoms from the chlorotrifluoroethylene molecule:
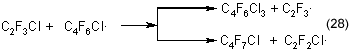
Dichlorohexafluoropropane formation may be explained by radical processes also:
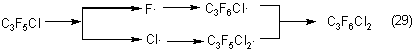
But the total concentration of these compounds in the pyrolyzate does not exceed 1% that makes possible to exclude the reactions of their formation from the scheme of the joint thermal decomposition of chlorodifluoromethane and dichlorofluoromethane.
Thus, the process may be represented by the following scheme describing formation of main products:
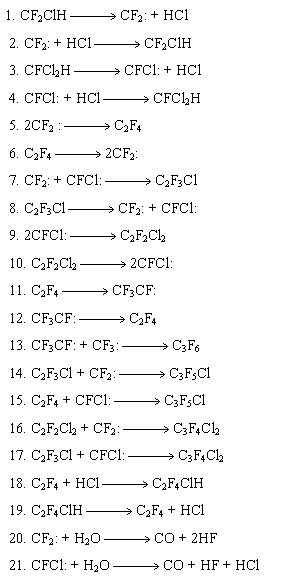
Experimental
For kinetic experiments there was used dichlorofluoromethane containing 99% of the main substance. A tubular reactor used in the experiments was 980mm length with inside diameter of 4 mm. The reactor was made of quartz containing a low content of aluminum oxide which catalyzed the reaction of dehydrofluorination competitive to the process. Thermal decomposition was carried out at dilution of the initial alkane with helium ( the He concentration was 99.0% by volume)
The experiments on joint thermal decomposition in the presence of aqueous vapor were carried out in the flow reactor made of the nickel-chromium corrosion-resistant alloy, having the diameter of 4 mm, work surface length - 500 mm. To prevent the cracking of the protective oxide film on the reactor surface, the heating and cooling of the reactor were performed in the linear regime with the speed of no more then 100 K per hour. The pyrolysis was carried out in the temperature interval of 973-1173 K at the vapor-freon molar ratio 10:1-1,5:1
The analysis of gas mixtures after purification from acid admixtures was made on a “Tsvet-100M” chromatograph with using a flame ionization detector and a thermal conductivity detector (helium as a gas carrier, 3m column length, 2mm column diameter, 293-323K column temperature, tricrezylphosphate ( 15% on silochrome-80)).
The identification of the pyrolysis products was made by a chromatomass-spectrometry method on a HP-5995 instrument ( 70eV energy of ionizing electrons, 553K separator temperature, 423 temperature of the ion source)
The mass-spectra of some products of joint pyrolysis of chlorodifluoromethane and dichlorofluoromethane are given below. The mass-spector describe the peaks with the respective intensity of more then 2% of the maximum peak, starting with m/e=35.
(m/e, (I,(I, %))).
Dichlorofluoromethane -102 (6), 69 (32), 67 (100), 48 (6), 47 (8).
Chlorodifluoromethane -86 (3), 85 (3), 69 (5), 67 (16), 66 (2), 51 (100), 50 (6), 47 (2), 40 (6), 37 (3).
Chlorotrifluoroethylene - 118 (34), 116 (100), 99 (9), 97 (27), 87 (10), 85 (31), 81 (18), 68 (11), 66 (33), 47 (19).
Tetrafluoroethylene - 101 (1), 100 (60), 82(2), 81 (100), 69 (4), 62 (2), 50 (40), 43 (2), 31 (50).
1,2-difluorodichloroethylene - 136 (10), 134 (61), 132 (100), 103 (9), 101 (14), 97 (8), 84 (37), 82 (53), 66 (14), 47 (21).
Chloropentafluoroethylene - 137 (11), 135 (34), 119 (53), 100 (4), 87 (30), 85 (100), 69 (70), 66 (5), 50 (25), 47 (4).
1,1,2-trifluorotrichloroethane - 155 (7), 153 (39), 151 (61), 116 (13), 105 (10), 103 (69), 101 (100), 87 (16), 85 (48), 66 (24).
1,2-difluorotetrachloroethane - 171 (21), 169 (63), 167 (70), 134 (13), 132 (21), 119 (41), 117 (42), 103 (67), 101 (100), 47 (40).
Tetrachloro-1,1-difluoroethane - 171 (30), 169 (9), 167 (100), 132 (22), 121 (30), 119 (91), 117 (91), 85 (38), 82 (30), 47 (52).
dichloro-1,1,2,2,-tetrafluoroethane - 151 (2), 137 (17), 135 (52), 103 (6), 101 (9), 100 (6), 87 (33), 85 (100), 69 (4).
Chloro-1,1,2,2-tetrafluoroethane - 135 (16), 119 (8), 117 (24), 101 (100), 87 (30), 85 (92), 69 (46), 67 (88), 51 (97).
dichloro-1,1,2,3,3,3-hexafluoropropane -1 201 (9), 155 (7), 153 (40), 151 (64), 134 (24), 132 (40), 103 (13), 101 (18), 85 (37), 69 (100).
Hexafluoropropene - 151 (2), 150 (31), 132 (3), 131 (100), 101 (3), 100 (42), 93 (8), 81 (17), 74 (4), 69 (96), 62 (6), 50 (15), 31 (30).
Chloro-1,1,2,3,3-pentafluoropropene - 168 (3), 166 (11), 149 (47), 147 (16), 131 (100), 116 (5), 93 (13), 85 (10), 81 (11), 69 (74).
Chloro-1,2,3,3,4,4,4-heptafluorobutene -1 - 216 (3), 181 (9), 149 (7), 147 (20), 132 (3), 131 (100), 112 (3), 93 (13), 85 (6), 69 (18).
References
1. Locke E.G., Brode W.R., and Henne A.L. Fluorochloroethanes and Fluorochloroethylenes. // J. Amer. Chem. Soc. – 1934. – Vol. 56 – No. 8 – P.1726-1728.
2. US Pat. N 2590433. Dechlorination of a halocarbon containing chlorine. / Blum O.A., Bayonne N.J.
3. Pat. N 79- 115305 Japan. Chlorotrifluoroethylene. / Terakawa A. et al
4. US Pat. N 3073870. A process for preparing trifluorochloroethylene. / Marquis D. M.
5. Avtorskoe svidetel'stvo SSSR № 166674 / SHevchuk V.U., Faragash M.B. (1964)
6. Nikolaev A.A., Rozhdestvenskaya O.V., Barabanov V.G. / Issledovanie sovmestnogo termicheskogo razlozheniya diftorhlormetana i ftordihlormetana // Tez. dokl 2 Mezhd. konf. «Khimiya, tekhnologiya i primenenie ftoroorganicheskih soedinenij v promyshlennosti» - SPb – 1997-1s.
7. Norton F.G. Rates of Thermal Decomposition of Chlorodifluoromethane and Dichlorodifluoromethane. // J. Refrig. Eng.-1957.-Vol.65.-No 9.-P.33-34.
8. Íisazumi M. Synthesis of fluororesin monomers. Accelerating effects of diluents on the pyrolysis rates of chlorodifluoromethane. // C. A.-1978.-Vol. 89.-ref. 2215766n.
9. Nefedov O.M., Ivashchenko A.A. Vysokotemperaturnyj sintez arilhloridov i arilftoridov cherez digalokarbeny. // Izv. AN SSSR, ser. khim., 1968, № 2, s. 446-448
10. Gozzo F., Patrick C.R. The Thermal Decomposition of chlorodifluoromethane. // Tetrahedron.-1966.-Vol. 22.
11. Barabanov V.G.‚ Volkov G.V., V'yunov K.A., Maksimov B.N. Vysokoehnergeticheskie protsessy prevrashchenij poliftorhloralkanov. VIII. Obshchie zakonomernosti termicheskih prevrashchenij hlorpoliftoralkanov. // Zhurn. obshchej khimii, 1990, T. 60, V. 5, S. 1156-1159
12. . Barabanov V.G.‚ Volkov G.V., V'yunov K.A., Maksimov B.N. Vysokoehnergeticheskie protsessy prevrashchenij poliftorhloralkanov. VIII. Obshchie zakonomernosti termicheskih prevrashchenij hlorpoliftoralkanov. // Zhurn. obshchej khimii, 1985, T. 55, V. 4, S. 868-871
Fluorine Notes, 2002, 22, 5-6
