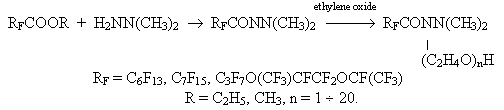Fluorine Notes, 2002, 22, 3-4
Synthesis of fluorine-containing surfactants derived from dimethylhydrazine.D.Ya.Kasaeva, S.E.Gonek, E.I Cherkas, E.N. Novozhilov, B.N. Maximov We have developed a method to produce fluorine-containing amino-imide surfactants derived from perfluoromonocarbonic acids and N,N-dimethylhydrazine , that was conditioned by necessity of N,N-dimethylhydrazine utilization after expiration of its warranty lifetime. Besides, we studied methods of purification of the surfactants produced and measured their physical and chemical characteristics. Amyl ethers of perfluoroheptanoic, perfluorononanoic acids and amyl ethers of hexafluoropropene oxide trimer were chosen as the source material for synthesis of new uorine-containing surfactants , i.e. for N,N-dimethylpolyethoxyaminoimides of perfluoromonocarbonic acids. The method consists in interaction of amyl ethers of perfluorocarbonic acids and trimer of hexafluoropropene oxide with N,N-dimethylhydrazine in a medium of polar solvents ( ethanol and isopropanol) under adiabatic conditions ( the reaction mass temperature was kept on the account of the reaction heat efficiency) followed by polyethoxylation of the produced aminoimide of gaseous ethylene oxide (EO):
To increase the yield of the end product the following process indices were determined: the temperature condition and concentration of reagents. The heat efficiency of the reaction of interaction of alkyl ethers of perfluoroheptanoic, perfluorononanoic acids and trimer of hexafluoropene oxide with N,N-dimethylhydrazine was measured by means of differential isothermal thermometry and was 119 kJ/mole. It was sufficient to form aminoimide. The process temperature was kept by the rate of feeding N,N-dimethylhydrazine. The choice of the optimal temperature in the stage of amidation (20-30oC) was caused by the fact that at a higher temperature values ( the upper limit) the aminoimide is gummed whereas at a temperature below 20oC the yield is reduced considerably. On completion of dropping N,N-dimethylhydrazine the aminoimide produced was aged for some time more at mixing. The aminoimide structure has been confirmed by the data of elemental analysis and IR-spectroscopy. The temperature in the stage of polyethoxilation was kept within a range of 30-45oC by means of water bath because at a temperature below 30oC absorption of ethylene oxide stopped and at a temperature above 45oC the aminoimide was resinified. As a result of the study of the reaction concentration parameters the optimal ratio of the ether and N,N-dimethylhydrazine was chosen as 1:1,2. An excess of N,N-dimethylhydrazine was a catalysis in the polyethoxylation process. The amount of ethylene oxide absorbed in the process was determined by a necessary oxyethylation degree for every concrete sample. Vacuum distillation was chosen as a method for isolation and purification of the end product. The solvent and excess amount of N,N-dimethylhydrazine were removed at a temperature of 60-70oC and pressure of 30 Torr. The yield of the end product reached 95%. The content and structure of the product synthesized has been confirmed by the data of elemental analysis and IR-spectrometry. The oxyethylation degree was determined according to an overweight and ascertained by PMR spectroscopy method comparing integral intensities of proton signals of the oxyethyl group with appropriate integral intensities of proton signal of two methyl groups of N,N-dimethylhydrazine used as a catalyst with strictly specified concentration. The physical and chemical data of new fluorine-containing surfactants has been received. The products synthesized were viscous waxy substances of a color from straw to dark brown with a density within a range of 1.3-1.5 g/cm3, well soluble in water. MW was equal ~ 726 for polyethoxyiminoamide of perfluoroheptanoic acid ( n=5). A rather enlarged samples were produced of the following substances: N,N-dimethylpolyethoxyaminoimide of perfluoroheptanoic acid with the oxyethylation degree n = 3,4,8,9; N,N-dimethylpolyethoxyaminoimides of perfluorononanoic acid with n = 5,8,10,16 and N,N-dimethylpolyethoxyaminoimides of trimer of hexafluoropropene oxide with n = 3,5,8,12.5. We developed a special reactor for the oxyethylation process which made possible to increase the contact time of gaseous ethylene oxide with the reaction mass. The reactor construction allowed controlling the process not by the overweight of the reaction mass but visually by the growth of the reaction mass height. The reaction was a two-tubule narrow cylindrical vessel equipped with a barometer for feeding gaseous ethylene oxide under the reaction liquid layer and with a thermocouple well. The surface tension of aqueous solution contained 0.36 wt.% of the active substance reached 21 mN/m maximum. For the same solution the foam ratio was 6 minimum and the foam stability was 6.5 min minimum. In future we intend to continue the study on development of new fluorine-containing surfactants derived from perfluorooxycaprylic acid and perfluoroalkansulfo acids of general formula
RFZNQ, Thus as a result of the studies carried out the method of synthesis of fluorine-containing surfactants of high efficiency has been developed for their application in fire-fighting compositions. The synthesis method was the interaction of perfluorocarbonic acids with N,N-dimethylhydrazine followed by ethoxylation. |
Fluorine Notes, 2002, 22, 3-4