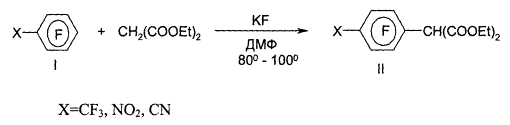
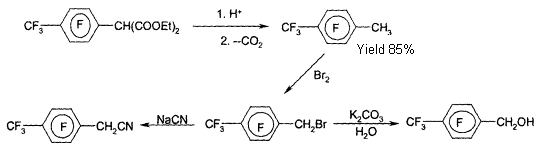
Dear Authors!
We would like to inform you that starting from the second issue of the journal, a mandatory requirement for providing translations of articles will be enforced. When submitting materials for publication, the original article must be accompanied by its translation. Please consider this requirement when preparing your scientific works for the journal.
Dear Authors
We would like to inform you that starting from the second issue of the journal, a mandatory requirement for providing translations of articles will be enforced. When submitting materials for publication, the original article must be accompanied by its translation. Please consider this requirement when preparing your scientific works for the journal.
Dear Authors
We would like to inform you that starting from the second issue of the journal, a mandatory requirement for providing translations of articles will be enforced. When submitting materials for publication, the original article must be accompanied by its translation. Please consider this requirement when preparing your scientific works for the journal.
Attention! All scientists, students & PhD candidates!
P&M-Invest continue promotion!
We offer our products as free sample, subject to the publication of their results in our journal "Fluorine
notes". The compounds are provided in the quantity 5-20 g (not more than 3 compounds one-time). The
research results, obtained based on each sample, should be published within 6 months. Only "products from stock"
are involved into this promotion.
Sale
SIA "P&M-Invest" Ltd is running the New Year Sale of our stock products from 22nd of October to the end of the
year.
If you buy the products at the amount 5 000 Euro, you will get 5% discount, at the amount 10 000 eur
-10 % discount , and at the amount 15000 - 15 % discount.
Dear Authors !
In April 2019 Fluorine Notes received the
results of the initial review by Clarivate Analytics for the admission
of our Journal into Web of Science database.
All the remarks will be taken into account and corrected in the nearest time. We hope to achieve the anticipated result to 2020.
Hereby, we are looking forward to receive new articles for our Journal from you !
Russian-Japanese "Fluorine seminar" in Novosibirsk
The Russian-Japanese "Fluorine seminar"was held in N.N. Vorozhtsov Novosibirsk Institute of Organic
Chemistry of Siberian Branch of Russian Academy of Sciences (NIOCh SB RAS), on 17th of December,
2019. There were made presentations by number of Russian scientists from NIOCh SB RAS: Prof.
Bardin V, Prof. Malikhin E., Prof. Tretyakov E. and Japanese scientists: Prof. Yamazaki from
University of Toyo, Prof. Shibata from the Technollogical Institue of Nagoya. Also there were made presentations by
the Senior employees of innovation business of Japan from
Daikin and Asahi Glass. They acquainted the audience
with the perspectives directions of organofluorine
compounds'application in these companies.

Abstracts of some reports will be published in the next issue of the journal.
Attention! All scientists, students & PhD candidates!
P&M-Invest continue promotion!
We offer our products as free sample, subject to the
publication of their results in our journal "Fluorine notes". The
compounds are provided in the quantity 5-20 g (not more than 3 compounds
one-time). The research results, obtained based on each sample, should be
published within 6 months. Only "products from stock" are involved
into this promotion.
Dear authors !
The results of the contest for the best publication 2016 -2017 will be summarized in May 2018.
Attention! All scientists, students & PhD candidates!
P&M-Invest offers “products from stock” as free samples (not more then 3 substances) in the quantity 5-20g, subject to the publication of their results, obtained based on each sample, in our journal “Fluorine notes” within 6 months. The promotion is valid within 2017.
Note: The delivery of free of samples is paid by receiver.
Dear readers and authors-to-be!
The Editorial Board would like to announce a contest for a best publication 2016-2017 in our Journal :
One First Prize - 2000 Euro
Two Second Prizes – each equals 1000 Euro
The decision on rewarding will be taken by conclusion of the vote of Editorial Board Members in the end of 2017.
It is our pleasure to announce that the 11th "All-Russian Fluorine Chemistry Conference"(F-2016) will be held in Moscow (Russia) from the 26th until the 30th of June 2016. More information on the web-site fluorine.moscow.
On-line registration is available until 31 of March.
Information Letter. First Circular.