Fluorine Notes, 2001, 19, 3-4
Synthesis of 2-perfluoroalkyl-5H-[1,2,4]-triazolo[5,1-b][1,3-]thiazines.
N.A.Danilkina, S.V.Vershilov, L.E.Mikhailov, L.M.Popova1
Saint-Petersburg chemical and pharmaceutical academy 14, prof.Popov str., Saint-Petersburg, Russia
1 RSC “Applied Chemistry”, 14 Dobrolubov str., S.-Petersburg, Russia
A few works describing methods to synthesise thiazines using different derivatives of acetylenecarbonic acids have been published by today. It is well known that condensation of heterocyclic compounds, azoles in particularly, containing a thioamide fragment with acetylene derivatives results in formation of 1,2-thiazines [1]. We tried to apply this method for building bicyclic thiazine systems containing perfluoroalkyl substituents because perfluoroalkyl derivatives of heterocycles have been attracted more and more attention recently [2]. That is due to changes in chemical properties and biological activity that arise when perfluoroalkyl groups are introduced.
A reaction of cyclization of 2-mercapto-5-perfluoroalkyltriazoles (2a,b) [3] with methyl ether of phenylpropionic acid in the presence of sodium methylate gives appropriate 2-perfluoroalkyl-5H-{1,2,4}-triazolo[5,1-b][1,3-]thiazines (3a,b).
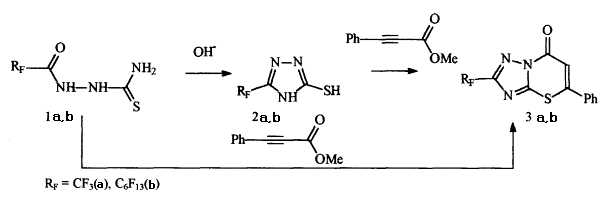
We made an attempt to get compound 3b in a counter synthesis, i.e. by a way of interaction of perfluoroacylthiosemicarbazide (1b) with methyl ether of phenylpropionic acid followed by cyclization of thiazine obtained in the presence of alkali. To our surprise, the only product of the reaction between perfluoroacylthiosemicarbazide ( 1b) and methyl ether of phenylpropionic acid in methanol at boiling during 2.5 hour was triazolothiazine(3b), whereas cyclization of perfluoroacylthiosemicarbazide (1 a,b) to perfluoroalkylmercaptotriazole (2 a,b) runs under more severe conditions ( boiling during 3 hours in 40% water solution of KOH) [3].
The structure of compounds obtained (3a,b) has been confirmed by NMR- and mass spectra. The 1H NMR spectra of compounds 3a,b contain a singlet of C5H protons of the thiazine ring (7.48 ppm) and a multiplet assigned to the benzene ring protons (7.63-7.9 ppm). The 13C NMR spectra show signals of carbon atoms of the thiazine and triazole cycle (150.3-155.0 ppm) and the C5 signal moves upfield in comparison with other signals of carbon atoms of the thiazine ring, there are also signals of carbon atoms of the phenyl radical (127.0-132.3 ppm) and a weak multiplet of carbon atoms of the perfluoroalkyl radical (110-120ppm).
The mass spectrum of compound 3b shows a peak of molecular ion M+ with m/z 547.
2-Trifluoromethyl-5H[1,2,4]-triazolo[5,1-b]-7-phenyl[1,3]thiazine (3a). 1.2mmol of methyl ether of phenylpropiolic acid and one drop of 5% solution of sodium methylate in methanol were added to a solution of 1 mmol of 2-mercapto-5-trifluoromethyl-1,2,4-triazole (2a) in 10 ml of methanol, the mixture was boiled for 6 hours, the solvent was then removed at reduced pressure (20 torr), the residue was re-crystallized from heptane. Yield of 71%, melting temperature of 140-142oC, Rf 0.57 (acetone-hexane, 1:4). 1H NMR spectrum (DMCO-d6),δ,ppm:7.47 s (1H, CH) 7.75m(5H,Ph).
13C NMR spectrum (DMCO-d6), δ ppm: 114.75, 127.02, 129.70, 132.30, 133.20, 150.36, 151.60, 154.16, 155.00. Found, %: C 48.5; H 2.0; N 14.1; S 10.8. Anal. Calcld for C12H6F3N3OS, %: C 48.1, H 2.7, N 13.7, S 11.8.
2-Perfluorohexyl-5H-[1,2,4]-triazolo[5,1-b]-7-phenyl[1,3]-thiazine (3b)
a) 1.2 mmol of methyl ether of phenylpropiolic acid and one drop of 5% solution of sodium methylate
in methanol were added to a solution of 1 mmol of 2-mercapto-5-perfluorohexid-1,2,4-triazole (2b)
in 10 ml of methanol, the mixture was boiled for 6 hours, the solvent was removed at reduced pressure
(20torr), the residue was re-crystallized from a small quantities of ethanol. Yield 68%, melting
temp. 125-128oC, Rf 0.71 (acetone-hexane, 1:4). 1H NMR spectrum
(DMCO d6), δ ppm: 7.48 s(1H,CH) 7.77m(5H,Ph). 13C NMR spectrum (DMCO-d6),
δ, ppm: 114.81,127.04, 129.72, 132.30, 133.23, 150.33, 151.20, 154.11, 155.00. MS [M+] : m/z 547.
Found,%: C 49.3; H 1.4; N 10.1; S 7.7. Calcld. for C17H6F13N3OS,
%: C 48.9; H 1.9; N 10.2; S 7.3.
b) 1.2 mmol of methyl ether of phenylpropiolic acid was added to a solution of 1 mmol of thiosemicarbazide of perfluoroheptanic acid (16) in 10 ml of methanol, the mixture was boiled for 6 hours, the solvent was removed at reduced pressure (20torr), the residue was re-crystallized from a small quantities of ethanol. Yield 79%.
1H NMR and 13C NMR spectra of the substances in DMCO-d6 were recorded on a Broker AM-500 spectrometer (working frequency was 500.17 and 83 mHz respectively). The mass spectrum were obtained using a MX-1321 instrument with direct input at a temperature of inlet of substances into the ion source of 140-300oC, the energy of ionizing electrons was 70 eV. The control over the reaction and purity of the products obtained was carried out by TLC method on Silufol UV-254 plates.
References
1. Janietz P., Goldman R., Rudolf W.D. // J. f. Pract. Chemie. 1988. Bd 330(4) p.607
2. Isikawa N., Kobayasi E., Ftor. Khimiya i primenenie: Per s yap. / pod red. A.V. Fokina. – M.: Mir, 1982. – 276 s.
3. Sintez 3-perftorzameshchennyh 1,2,4-triazolil-5-aminov i -5-tiolov. / S.V. Vershilov, L.M. Popova, V.E. Mungalov, N.A. Ryabinin // Zhurn. prikl. Khimii. – 1994. –t. 67. – v. 7. – s. 1124-1126
Fluorine Notes, 2001, 19, 3-4
