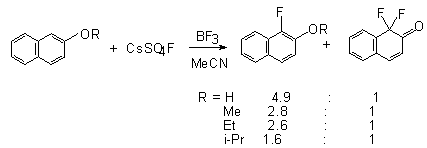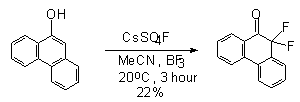This paper summarizes and systemizes up-to-date information on synthesis of organofluorine
compounds of different classes with use of new reagents as fluorine carriers
including organic compounds containing O-F bonds (hypofluorites of perfluorinated
alcohols and carbonic acids) and cesium fluorooxysulfate. Fluorinating ability
of these reagents is comparatively analyzed in dependence on their structure
and the solvent nature. A feasibility to fluorinate unsaturated organic,
heterocyclic and hetero-organic compounds is discussed. Matters of a mechanism
of fluorination with compounds containing O-F bonds are examined. Specific
features of carrying out the processes of fluorination, their merits and
demerits in comparison with reactions using elemental fluorine, xenon difluoride
and other fluorinating agents are revealed. Availability of methyl- and tret-butylhypofluorites
as reagents able to introduce the alkoxy- group into unsaturated organic
compounds and their opportunities are shown. Examples of application of HOF/MeCN
system as an oxidizer of unsaturated compounds to carry out processes of
epoxidation and hydroxylation of olefins are under review. This oxidizer
advantages, its specific peculiarities and application in organic synthesis
are discussed.
Conclusion
References
5.1. Fluorinating properties of cesium fluorooxysulfate containing the O-F ionic bond .
After the development of the method to produce cesium salt of fluoroxysulfate
by Appelman the interest to this compound is steadily increasing [92-94].
Appelman isolated and completely described CsSO4F and RbSO4F
[92,93]. The structure of the rubidium salt was analyzed by the X-ray
structure analysis [95]. The oxidizing properties of CsSO4F
were described in [96,97]. Zoopan’s team in Yugoslavia executed the main
work. Cesium fluoroxysulfate CsSO4F is the most stable among
the class of compounds containing the O-F bond and may be successfully
used in practice provided safety regulations. The organic chemistry of
CsSO4 has been intensively studied for the last ten years
that brought to the understanding of uniqueness of this ionic electrophilic
fluorinating reagent used at present for selective fluorination of organic
compounds (see 98,99). The reactions with aromatic, heterocyclic compounds
[93,94,99], organometallic derivatives [100],  -diketones [101], alkenes [102,106] etc. have been studied.
-diketones [101], alkenes [102,106] etc. have been studied.
Its reactions with organic substrates depend strongly on the type of the
organic molecule and the character of a functional substituent in it.
It was determined that even small changes in the substrate nature and
reaction conditions are sufficient for another way of the process passing.
Capabilities of this reagent have not been determined completely but
even the existing data allow characterizing it as a very perspective
and convenient reagent in laboratory practice. It is important that it
may be successfully used for the synthesis of mono fluoro-derivatives
of the aromatic series among which compounds the high biological activity
have been found.
5.1.1. Aromatic and unsaturated compounds in the reactions with cesium fluorooxysulfate.
The investigation of the reactions of aromatic compounds with CsSO4F
have revealed the character of behavior of this fluorinating agent .
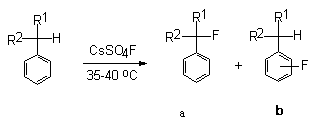
It has been found that CsSO4F in acetonitrile at 35oC
is a mild reagent fluorinating mono-alkylbenzenes to the benzene main
body and to the side chain ( Table 3)[49,107,108]. Functional orientation
of mono- and di-substituted benzene derivatives under the influence of
CsSO4F depends on the nature of the substituent in the benzene
ring and the process may be directed either to the fluorination of the
benzene ring itself or to involve the substituent [109]. In this process
the conditions are of great importance (Table 3). The process proceeds
regioselectively. When BF3 is used as a catalyst, the fluorination
affects only the benzene ring [101].
Table 3. Results of fluorination of monoalkyl-substituted of benzene under the influence of CsSO4F [101]
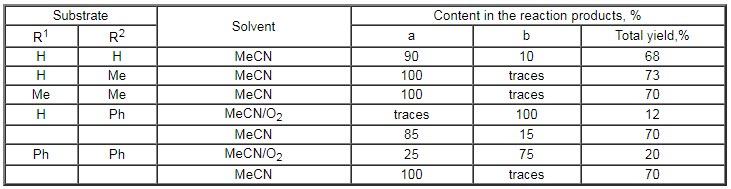
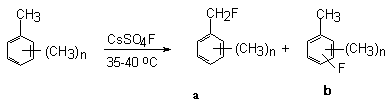
The presence of some alkyl groups in the benzene ring does not change the character of the forming products (Table 4) [109]. The fluorination of di- and trialkylbenzenes takes place according to the same scheme: compounds with fluorine are formed both in the benzene ring and in the alkyl fragment (Table 4)[109].
Table 4.
Fluorination of dialkyl- and trialkyl-derivatives of benzene under the
influence of CsSO4F in MeCN (60% excess of CsSO4F)
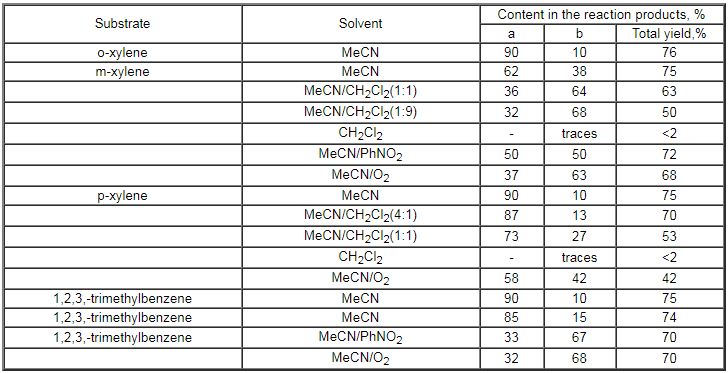
In case of polycyclic aromatic compounds the yield of the fluorination products
is slightly less in comparison with the benzene derivatives though the
ratio of the isomeric products remains the same and an increase of the
ortho-isomer takes place [108].
In case of polyaromatic compounds (naphthalene[103,109], phenanthrene, pyrene
[108]) mixtures of isomeric mono fluoroderivatives and also difluoroderivatives
can be formed [101].
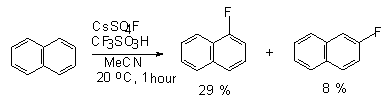
Zoopan used CsSO4F for the fluorination of nonactivated polyaromatic
compounds, naphthalene, phenanthrene and pyrene, at room temperature
for 4 hours [108].
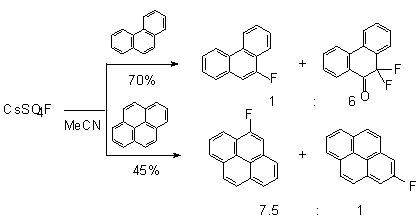
In case of the fluorination of pyrene, there is a need in a solvent in which
pyrene would be easily dissolved. In this case 1-fluoro- and 4-fluoropyrones
are formed in the ratio of 7.5:1 in a total yield of 40-45%.
Nonbenzene aromatic derivatives, porphyrins, react with CsSO4F
to form 5-fluoropophyrins together with di-,tri- and tetrafluoro derivatives
[111].
The processes of addition and elimination take place in the reaction of substituted
benzene and norbornene at an excess of CsSO4F in methene chloride.
It has been found that using CsSO4F in alcohols it is possible
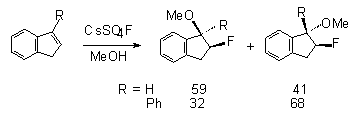
to fluorinate successfully conjugated olefins , for example indene, acenaphthylene,
stilbene and substituted phenanthrene. Stereoselectivity of the reaction
of 1-phenyl-1-benzocyclene with CsSO4F in alcohols was studied
in papers [105,112,113]. The results are given in Table 5.
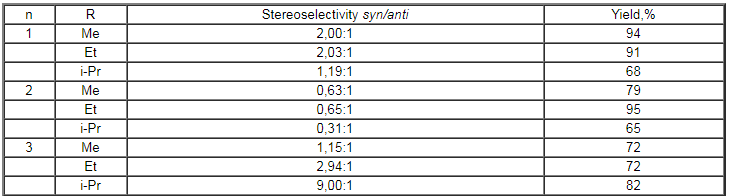
Table 5. Influence of structure of benzocyclene and the nature of ROH alcohol on stereoselectivity of the formation of vicinal fluoroethers in the reaction with CsSO4F [105]
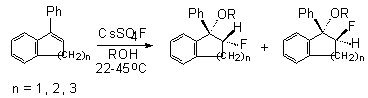
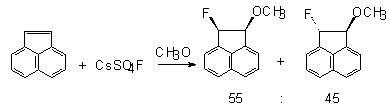
The stereochemistry of the process was also studied in fluorination of acenaphtene, stilbene, indene and 1-phenylidene with CsSO
4F. Thus, the interaction of CsSO
4F with acenaphthylene leads to the formation of products of fluoromethoxylation syn:anti in a ratio of 55:45. In case of elemental fluorine at –78
oC the ratio is 35:11 and it is 16:84 for xenon difluoride.As
it is evident from the data of table 5, the transition from
methyl to isopropyl alcohol results in a considerable increase
in the content of syn-isomer in case of the 7-member cycle,
that points to an increase of stereospecificity of the process,
whereas it is not observed for small cycles.
If alkyl-substituted benzenes in a reaction with CsSO4F form mixtures
in different ratios of isomeric mono-fluoro-derivatives, then oxy- and
alkoxy-derivatives give ortho-substituted fluorobenzenes preferably.
The nature of the alkoxy-group influences the ratio of isomeric ortho-
and para- fluoroalkoxybenzenes [49,107,108].Table 6 shows the data on
fluorination of anisole with various fluorinating reagents.
Table 6. Results of fluorination of anisole with different reagents
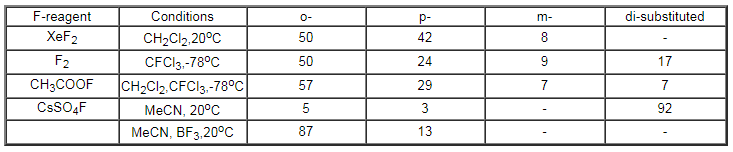
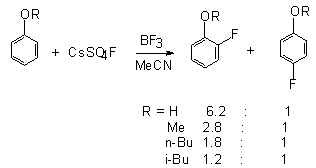
It is evident from the data that only CsSO4F falls out from a
common pattern and gives practically completely ortho-isomeric product.
It is most preferable to carry out such processes in the presence of
polar solvents and catalysts, strong proton acids or BF3.
The yield of the products is 70-80%[114].
Appelman and collaborators studied the effect of acid catalysis (HF,H2SO4,
BF3, CF3SO3H, FSO3H and SbF5-FSO3H)
on the reaction of CsSO4F with toluene, nitrobenzene and naphthalene
in acetonitrile [110,114]. In a general case an increase in acidity of
the catalytic system results in a catalytic effect increase. These results
are interpreted in terms of electrophilic fluorination catalyzed with
acids. A similar picture takes place in the interaction of CsO4F
with 1-naphtol and 1-alkoxynaphthalene in the presence of BF3 as a catalyst [49]. The yield of alkoxynaphthalenes is above 50%.
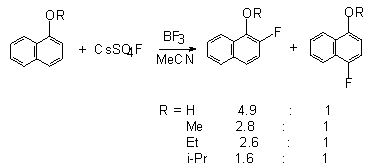
At the same time 2-naphtol and 2-alkoxynaphthalenes in the reaction with
CsSO4F in the presence of BF3 give 1,1-difluoro-2-oxa-1,2-dihydrohaphthalene
together with the fluorination product in the ortho-position in a total
yield of 60-80% [114]. For the first time the tendency to form
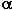 -difluoroketones was shown exactly in the reaction of CsSO4F
with 1-alkoxy- and 2-alkoxynaphthalenes [100,107].
-difluoroketones was shown exactly in the reaction of CsSO4F
with 1-alkoxy- and 2-alkoxynaphthalenes [100,107].
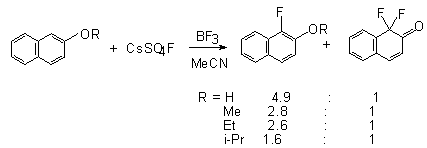
In a number of cases difluoro derivatives are the main products of the reaction
[102].
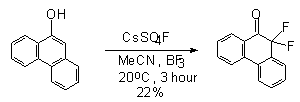
9-Acetamidophenanthrene under the influence of this reagent gives 10,10-difluorophenanthrene
9(10H)-one in 22% yield , whereas 9-hydroxy- or 9-methoxy- and 9-acetoxy-derivatives
give 9-fluoro-1-hydroxyphenanthrene or 10-fluoro-9,9-dimethoxy-9,10-dihydrophenanthrene
[108].The fluorination of aniline with CsSO4F results in the
formation of a mixture of 2- and 4-fluoroanilines. The use of the reagent
labeled with19F gives a possibility to obtain important diagnostic
preparations for medical purposes.
The reaction of styrene with CsSO4F in acetonitrile gives two
vicinal fluorosulfates with anti-Markovnik regioselectivity[115].
Norbornene under the influence of CsSO4F reagent gives a mixture
of 7-fluoro-nortricyclane and 7-syn-fluorononborn-2-ene [104].
The interaction of CsSO4F with unsaturated compounds is in general
an occurence of several reactions: substitution of the hydrogen atom
at the multiple bond with fluorine, addition to the double bond, conjugated
fluorination with participation of the external nucleophile which source
is a solvent. The completeness of the latter is influenced by the quantitative
ratio of solvent/substrate [115,116]. In methyl alcohol fluoromethoxylation
of the multiple bond takes place [102,103]. These ways are exhibited
in the mentioned below scheme and data of Table 7.
Taken 1,2-diphenylethylene (table 8) and 1,1-diphenylethylene (table 9) as
an example, it is possible to compare the data on stereochemistry of
the fluorination process with cesium fluorooxysulfate and other reagents.
As it is obvious from Table 8 [108], CsSO4F does not take
any representative place in this series. In the reaction with (E)-stilbene
it gives preferably syn-isomer whereas the mentioned fluorinating agents
with (Z)-stilbene give an identical ratio of syn:anti isomers. Only trifluoroacetylhypofluorite
promotes in fact selective process.
As we repeatedly noted, the reaction of CsSO4F with unsaturated
compounds results either in vinyl fluorides or in products of conjugated
fluorination with participation of external nucleophiles.
The authors of paper [110] managed to determine conditions under which 1,2-addition
of CsSO4F to the multiple bond occurs to form cesium salts
of fluoro-alkylsulfates. For the time being that is the only example
of simultaneous introduction of fluorine atom and nucleophilic sulfate
group into an organic molecule.
According to the usual scheme CsSO4F reacts with a group of olefins
studied by Zoopan: fluoromethoxylation of the multiple bond takes place
in the reaction of CsSO4F with alkenes in methyl alcohol [117,118].
A complex mixture of products is formed in fluorination of acetylene derivatives
with CsSO4F (table 10). So, the fluorination of 1,2-diphenylacetylene
in methanol gives two products: 1,1-difluoro-2,2-dimethoxy-1,2-diphenylethane
and 2,2-difluoro-1,2-diphenylethanone [55,118]. Thus, during the course
of the reaction, due to transformation of primary reaction products,
compounds containing the carbonyl group can be formed [55]. A share of
such compounds can be significant (Table 10) and the nature of the substituent
at the triple bond does not much affects the ratio of the reaction products,
for example in case of substituted phenylacetylene (R=H,Ph,t-Bu).
The authors of papers [109,119,120} developed a new method of regiospecific
introduction of a fluorine atom in reactions of CsSO4F with
benzyl alcohols and a-hydroxy-derivatives of aromatic compounds in acetonitrile
under rather mild conditions( the yield was 70-86%). In this case the
substituent at the benzene ring was replaced with fluorine to form respective
aldehydes. CsSO4F in reactions with aromatic and aliphatic
aldehydes in contrast to other fluorinating agents, xenon difluoride
for example, gives product of substitution of the proton at the carbonyl
group with formation of fluoroanhydrides of substituted benzoic and alkyl-carbonic
acids in a high yield [121]. The rate of these conversions is controlled
by the nature of the substituent in the benzene ring.
In case of xenon difluoride, the products of substitution of the carbonyl
group with two fluorine atoms are formed[122].
Primary aliphatic alcohols under effect of an excess CsSO4F give
fluoroanhydrides of aliphatic acids, whereas cyclic and acyclic secondary
alcohols under the influence of CsSO4F are converted to respective
ketones [123,124]. It should be noted that the presence of a radical
initiator, nitrobenzene for example, substantially reduces the yield
of the target products.
At the same time phenols are fluorinated on the benzene ring.
Cyclic secondary alcohols , for example 4-tret-butylcyclohexanol under action
of CsSO4F gives only 4-tret-butylcyclohexanone [125].
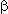 -Diketones under the influence of CsSO4F give a mixture of monofluoro and difluoroketones [126].
-Diketones under the influence of CsSO4F give a mixture of monofluoro and difluoroketones [126].
Benzophenone and 5,5-dimethylcyclohexa-1,3-dione under the influence of CsSO4F
also give 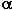 -fluoro-
and
-fluoro-
and 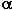 ,
,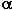 -difluorobenzophenones (in the ratio of 2,3:1) and 2-fluoro-3-hydroxy-5,5-dimethyl-2-cyclohexene-1-one
( in 66.5% yield) [100]. At the same time enoles of acetates of cycloalkanes
give as a rule
-difluorobenzophenones (in the ratio of 2,3:1) and 2-fluoro-3-hydroxy-5,5-dimethyl-2-cyclohexene-1-one
( in 66.5% yield) [100]. At the same time enoles of acetates of cycloalkanes
give as a rule 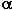 -fluorocycloalkanones
[100,126].
-fluorocycloalkanones
[100,126].
This property has been used to obtain fluorine-containing steroids. For example,
the synthesis of 2-fluoro-3-cholesterone was done by fluorination of
CsSo4F that was an important way to obtain 2-fluorovitamin D [127]. Similarly
16-fluoroestrone was produced [128].
to be continued
 -diketones [101], alkenes [102,106] etc. have been studied.
-diketones [101], alkenes [102,106] etc. have been studied.





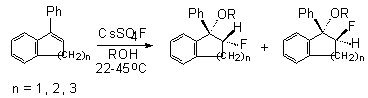





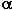 -difluoroketones was shown exactly in the reaction of CsSO4F
with 1-alkoxy- and 2-alkoxynaphthalenes [100,107].
-difluoroketones was shown exactly in the reaction of CsSO4F
with 1-alkoxy- and 2-alkoxynaphthalenes [100,107].