Fluorine Notes, 2001, 17, 3-4
Application of internal perfluoroolefins and perfluoroazaalkenes in synthesis of heterocyclic compounds with perfluoroalkyl groups.G.G. Furin* a , Ki-Whan Chi b * a Institute of Organic Chemistry, Russian Academy of Sciences, 630090 Novosibirsk, Russia b Department of Chemistry, University of Ulsan, Ulsan 680-749, South Korea The paper gives the data of the authors on synthesis of 3-7-membered and polycyclic heterocycles with one or several heteroatoms, which may make a basis and ground for semi-products for production of medicine and agriculture preparations and for new modern materials of wide field of application. It was shown that heterocyclic compounds with perfluoroalkyl groups could be predecessors for synthesis of biologically active substances, they are produced in ways based on reactions of binucleophilic reagents with internal perfluoroolefins and perfluoroazaakenes. Potentialities of building heterocyclic nuclei of different sizes (which structures were confirmed in most cases by data of X-ray diffraction analysis) in reactions with primary alkylamines (azetine and diazete), anilines (quinoline and qiunozaline), arylhydrazines(pyrazole), urea, acylamidine, arylamidine, guanidine ( pyrimidine and s-triazine), thiourea and its derivatives (thiazole) ethylendiamine (9-fluoro-5,9-bis(pentafluoroethyl)-6,8,8-tris(trifluoromethyl)-1,4-diazabicyclo[5.2.0]none-4,6-dien), 2-aminopyridine (2H-pyrido[1,2-a}pyrimidine, [1,3,5]-thiazine), benzimidazoline-2-thione ((2E)-2(1,2,2,2-tetrafluoroethyliden)3,3-bis(trifluoromethyl)-2,3-dihydrothiazolo[3,2-a] benzymidazole were shown on example of perfluoro-2-methyl-2-pentene and perfluoro-5-azanon-4-en . Main tendencies were determined in implementation of the approach that included generation of a new multiple bond and intermolecular nucleophilic cyclization. Influence of structural factors of a nucleophilic reagent on the direction of building a heterocyclic system was determined. Correlation of the reactivity of perfluoroolefin and factors determining generation of intermediate carbanions and elimination of fluoride ion was observed. Possible ways for application of new fluorine-containing heterocycles was shown. 1. Introduction Regioselective replacement of hydrogen with fluorine or with perfluoroalkyl group in a heterocyclic system influences much biological and physical properties of a molecule. As a result recently a number of papers aimed at development of methodology of synthesis of fluorine-containing heterocyclic compounds that uses methodology of formation of heterocycles on the account of double bond of perfluoroolefin and binucleophilic reagent has increased. Ability of fluoroolefins to react with nucleophiles distinguishes them from hydrogen-containing
analogues radically. It is pronounced in case of perfluoroolefins which chemistry
is developing intensively due to industrial production of key substances.
These properties of perfluoroolefins bring them closer to properties of activated
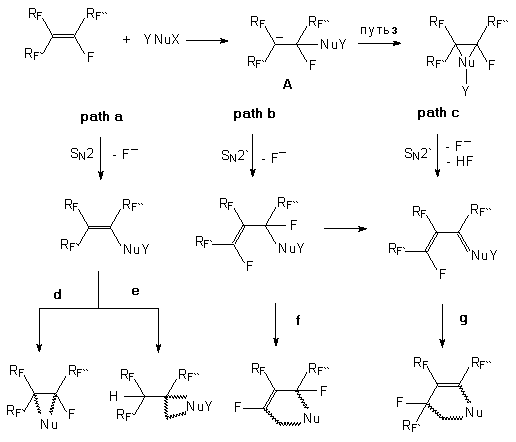
olefins of a type of High electrophilicity of perfluoroolefins is caused by combination of strong electron-seeking effect of fluorine atoms and CF3 groups which is intensified by ability of vinyl atoms of fluorine to effective conjugation with the C=C bond. The reactivity of perfluoroolefins is determined not so much by static distribution of electronic density ( and perfluoroolefins have a considerable positive charge on the carbon atoms of the C=C bond) as by dynamic properties of this distribution. Variety of reactions of perfluoroolefins is determined by mobility of fluorine atoms of the CF2 group, that is characteristic for fluoroolefins, and by possibility for further transformations of adducts due to, in particular, high CH-acidity of (CF3)2CH group. All this together with availability allows wide application of perfluoroolefins in organo-fluorine synthesis. It is known that an increase in fluorine atoms number in an organic molecule results in a substantial increase in positive charge on carbon atoms due to the difference in C and F electronegativity. This effect in its turn increases activity of fluorine atoms bonded with the C=C bond or C-F bond of an aromatic ring. That makes possible to get heterocyclic systems due to intermolecular nucleophilic cyclization on the account of the multiple bond of perfluoroolefin and the fluorine atom at the ortho-position of the perfluorinated benzene ring. The processes are well known , were the subject of wide investigations [1-7] and have become one of the most important and common method of synthesis of fluorine-containing benzo-heterocycles. There are used both nucleophilic reagents with two nucleophilic centers and heteronucleophiles which react with perfluorinated unsaturated compounds with generation of a new nucleophilic center that enters intermolecular nucleophilic cyclization. This methodology has great capabilities in obtaining new cyclic systems and in developing new approaches. Wide capabilities have been opened here both in synthesis of biologically active compounds and in plane of theoretical study of the nature of the carbon-fluorine bond. In case of internal perfluoroolefins and their derivatives an absolutely new possibility in formation of a heterocyclic ring appears due to the presence of CF2 fragments in the carbon chain [7]. Thus, an intermediate carbanion generated by interaction of the nucleophile with the multiple bond of the fluoroolefin may be stabilized with formation of both a primary multiple bond and new multiple bond. If there is a second nucleophilic center in the nucleophilic part or it is generated under the process conditions then intermolecular nucleophilic cyclization can affect the both multiple bonds resulting in formation of heterocycles differing in their structure and sizes. The nature of the heteroatom of the first nucleophile is of great importance because it takes part in formation and stabilization of the intermediate carbanion and determines the attack direction of the second nucleophilic center towards the carbon atom of both the primary and secondary multiple bond. The direction of such processes is determined by combination of several factors and allows to control purposefully the phase of heterocyclic system formation. That is why such processes are of the most interest and importance for perfluorinated compounds by virtue of the absence of such processes for olefins of hydrocarbon series and present a possibility to vary properties in different functional groups at the expense of introduction of fluorine atoms. It should be noted that in reactions of perfluoroolefins with nucleophilic reagents not only formation of a heterocyclic ring takes place but also perfluoroalkyl groups are formed at it. The key methods of synthesis of heterocyclic compounds with perfluoroalkyl groups are based on two types of chemical transformations [2,3,7]. The first type combines processes affecting the heterocyclic system in which a perfluoroalkyl group is to be introduced. These processes are realised mainly by substitutive fluorination of the already existing fragments or by direct introduction of the perfluoroalkyl group into the heterocycle. Development of convenient approaches and methods of direct perfluoroalkylation is one of important tasks of synthetic organic chemistry. This approach of synthesis of perfluoroolefins and their derivatives allows successful obtaining different heterocyclic compounds including biologically active ones. Besides, they are of importance for theoretical study of the C-F bond nature also based on experimental synthesis data. The analysis of the informative experimental data accumulated up to now seems insufficient [7]. The second type includes the process of formation of the heterocyclic system of blocks containing perfluoroalkyl groups or their fragments. Each of these types has its own advantages and disadvantages. Thus, if the first type uses perfluoroalkyl radicals and carbcations based on the developed methods of their generation ( thermolysis, photolysis, electrolysis, one-electron oxidation etc.) as reaction particles, the second type uses the processes of condensation of molecules with appropriate groups and nucleophilic reactions of perfluoroolefins. Development of a new effective synthetic methodology for selective introduction of a perfluoroalkyl group into organic molecules is an actual task connected with the search of new objects possessing biological activity, for making medicinal preparations , agrochemical substances, technical materials etc. Another the most interesting and important method could be reactions of perfluoroolefins with nucleophilic reagents. The peculiarity of chemical behavior of perfluoroolefins is in the process of preliminary addition to the multiple bond followed by elimination. Primarily there is addition of the nucleophile to the multiple bond with formation of carbanion A. Further transformations of carbanion A proceed in accordance with electron and space effects in dependence on the reaction conditions and existence of nucleophilic catalysis. Here several possible ways may be realized that leads to different reaction products (scheme1). It should be noted that nucleophilic catalysis has a general value in chemistry of compounds with electrophilic multiple bonds and is used, in particular, for dimerization and trimerization of activated olefins, keten-imines etc. The study of the factors influencing the processes of intermolecular nucleophilic cyclization and formation of cycles from systems containing double bonds carrying a labile fluorine atom is shown by the example of the reaction of internal perfluoroolefins and perfluoroazaalkenes with binucleophiles containing nitrogen, and the range of possible structural types of heterocyclic compounds is found to be wide enough. The heterocycles with one or several heteroatoms and having perfluoroalkyl substitutes are obtained at the expense of involving intermediate compounds if a double bond and a potential nucleophilic center on a substitute in it is available in nucleophilic cyclization. Reactions of perfluoroolefins with 1,3-bidentate nucleophiles in the presence of bases result in formation of different heterocycles with 5-, 6-, 7- and 8-memebered cycles and have been investigated rather widely in recent years.
Scheme1. If the charge on the carbon atom is stabilized in carbanion A at the expense of redistribution on neighboring perfluoroalkyl groups and
if the nucleophilic reagent has a big volume, then the double bond formation
becomes profitable in steric aspect. If the fluorine atom is at the carbon
If the functional group having a nucleophilic center is at the multiple bond
and possesses electron-donating properties then the attack towards the nucleophilic
center is directed to the So, under conditions of the presence of the multiple bond in the fluorinated molecule and possibility of generation of an anion center ( both in the heteroatom and in the carbon atom) in the functional group at this multiple bond due to intermolecular nucleophilic cyclization it is possible to obtain heterocyclic compounds with one or several heteroatoms and containing perfluoroalkyl groups. These new approaches point to the specific effect of fluorine atoms and the most important role of electron factors in hydrocarbon frame of the molecule in determination of the nucleophilic center attack direction and allow to reveal new, not specific for hydrocarbon analogues, reactions enriching the organic chemistry arsenal. The importance of these reactions is caused by availability of perfluoroolefins and the data obtained allow to transform them into a powerful instrument of modern organic synthesis. The reactions with different types of 1,1-, 1,2-, 1,3-, 1,4- binucleophilic reagents were assumed as a basis of systematization of the experimental data on the synthesis of heterocyclic compounds derived from perfluoroolefins and containing perfluoroalkyl groups. Though the general regularities of the reactions with nucleophiles are preserved, the main point of further transformations of primary products ( either adducts or products of replacement of functional groups at the internal multiple bond) is the influence of the introduced functional group containing a heteroatom. Here one may expect the principal differences in the influence of the nature of the nucleophilic reagent and implementation of the cyclization processes at the expense of new or existing nucleophilic centers in the molecule. Besides, an important aspect is a problem of isomerization under action of the nucleophilic agent of the primary internal olefin to terminal or internal one of a different structure. This can exert decisive influence on the structure of the heterocycle formed. In this report we give the data on the reactions of perfluoro-2-methyl-2-pentene (1) and perfluoro-5-azanon-4-ene (2) with a number of nucleophilic reagents in the presence of triethylamine and also we show the examples of formation of 3-7-membered and polycyclic heterocycles with a different number of heteroatoms. |
Fluorine Notes, 2001, 17, 3-4