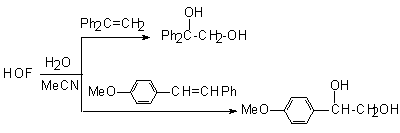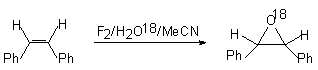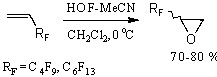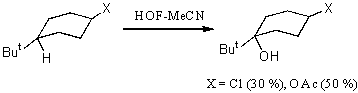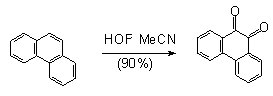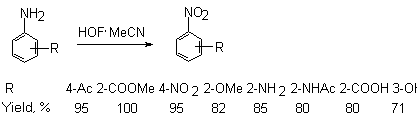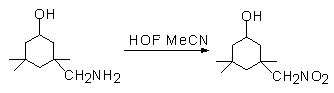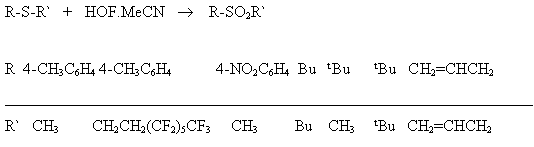Fluorine Notes, 2001, 16, 1-2
Some tendencies in application of reagents containing O-F bonds in organic synthesisG.G.Furin Novosibirsk Institute of organic chemistry named after N.N.Vorozhtsov, Siberian branch of the Academy of Science 9, Lavrentev ave., Novosibirsk, 630090, Russia Fax: 8-3832-344752 E-mail: furin@nioch.nsc.ru This paper summarizes and systemizes up-to-date information on synthesis of organofluorine compounds of different classes with use of new reagents as fluorine carriers including organic compounds containing O-F bonds ( hypofluorites of perfluorinated alcohols and carbonic acids) and cesium fluorooxysulfate. Fluorinating ability of these reagents is comparatively analyzed in dependence on their structure and the solvent nature. A feasibility to fluorinate unsaturated organic, heterocyclic and hetero-organic compounds is discussed. Matters of a mechanism of fluorination with compounds containing O-F bonds are examined. Specific features of carrying out the processes of fluorination, their merits and demerits in comparison with reactions using elemental fluorine, xenon difluoride and other fluorinating agents are revealed. Availability of methyl- and tret-butylhypofluorites as reagents able to introduce the alkoxy- group into unsaturated organic compounds and their opportunities are shown. Examples of application of HOF/MeCN system as an oxidizer of unsaturated compounds to carry out processes of epoxidation and hydroxylation of olefins are under review. This oxidizer advantages, its specific peculiarities and application in organic synthesis are discussed. Contents
|
Technological progress in many respects is determined by quality of applied materials. The most perspective way to give necessary and sometimes predictable properties to materials is modification of an organic molecule by means of introduction of elements differed from hydrogen into it. Just so new materials have been created for recent years. Thus, a replacement of hydrogen atoms with fluorine leads to new unusual properties of all the molecule as a whole, that allows to consider organofluorine compounds as new sources of potentially interesting and important materials for new branches of technology [1-4]. If earlier such compounds found an application as models to solve some theoretical questions of organic chemistry, at present a great interest in practical implementation of these new materials have been outlined that caused by requirements of medicine and technique. Their production is possible in the following ways: at first, by direct fluorine insertion into organic molecules that stimulates development of new ways to the realization of the fluorination process itself. Second, insertion of fragments containing fluorine atoms into organic molecules that is the most attractive and encouraging strategy in construction of new molecules with a set of predictable properties; third, formation of an organic molecule of several fragments containing the fluorine atoms [5]. Transformations in changes of physical and chemical properties caused by the introduction of the fluorine atoms into different parts of a molecule are very specific and are not found in many cases in organic derivatives of different elements. Just singularity, originality sometimes, of the new materials promotes development of new technologies and approaches in organic synthesis and makes prospects for the use of organofluorine compounds as new materials in an expanded area of application. The introduction of the fluorine atoms into an aromatic ring is an important task for the organic and pharmaceutical chemistry. The known methods to introduce fluorine into the aromatic ring are rigidly limited by the classic Balts-Schiemann reaction and decarboxylation of fluoroformates . The direct fluorination of organic molecules with elemental fluorine, characterized by explosive behavior and caused by high exothermicity of the process, requires special conditions. Fluorine is an extremely active gas with the dissociation energy of the F-F bond of 37 kcal/mol and is able to enter very different reactions. The use of low temperatures, dilution of fluorine with inert gas or carrying out the process in inert solvent allow in many cases to "tame" fluorine activity. Serious difficulties in synthesis of organofluoric compounds with elemental fluorine called into being various versions of fluorine introduction into organic molecules by means of fluorine "carriers". They are substances that possess a reduced oxidizing ability and allow carrying out fluorination processes under controlled conditions. In recent years a certain progress in development of routes to introduce one or two fluorine atoms into the benzene ring was outlined. These methods are based on electrochemical fluorination in melts of salts of metal fluorides or in anhydrous hydrogen fluoride [5]. But these methods have significant disadvantages that are determined in main by a low selectivity of the process of replacement of hydrogen atoms of the benzene ring because it is accompanied by addition to multiple bonds. As regards unsaturated organic substances, the situation is more complicated because all methods are not selective and a substantial destruction of an organic substrate molecule takes place. That was obviously became apparent in attempts of selective introduction of fluorine atoms into steroids, sugars and other natural substances. Therefore the task is not only to "tame" active fluorine possessing high oxidizing properties but also to develop new technologies of the fluorination process itself that include both creation of a selective fluorinating agent and make the fluorination process easier. Though the use of higher fluorides of transition metals and hydrogen fluoride made possible in a number of cases to introduce the fluorine atoms into organic molecules, the well-known experimental difficulties and complexity of the process itself, plurality of fluorination routes did not permit to use the process in fine organic synthesis, although a substantial successes of the method have been acknowledged in producing series of freons and other fluorohalogen-containing compounds [6]. The exchange reactions of chlorine atoms with fluorine under subjection to fluorides of alkaline metals are effective only for systems containing strong electron-seeking groups; it substantially constricts the frames of application of this method. Xenon fluorides possess universal fluorinating ability. But availability of the fluorinating agent, ecological compatibility and range of application did not find proper development due to the presence of foully highly explosive admixtures in xenon difluoride and intensity of the fluorination. Systems that have the fluorine atom, bound with a heteroatom, oxygen, nitrogen and sulfur, seem more perspective. Even the first steps in this direction have shown availability to use this route. In fact, monographs [5-9] and reviews [10-14] have displayed the examples of high-selective fluorinating reagents based on compounds containing the oxygen-fluorine bonds. There is direct evidence of progress in realization of these routes, especially in fluorination of heterocyclic compounds, steroids, sugars and natural substances. After the pioneer work of Barton et al. [15,16] on reactions of fluorooxytrifluoromethane with organic substrates the number of examples of synthesis of fluorine organic compounds with the use of compounds, containing a O-F bond, increases continuously; their application in fine organic synthesis is not exotic now but it is a recognized process realized in industry. It may be affirmed that there is a tendency in revision of the concept of the elemental fluorine use in fluorination processes and in application of a number organic compounds containing the active fluorine atom at a heteroatom and it is possible to replace elemental fluorine as a reagent, though its important role as a system and fluorine source have been remained.
Though the reagents containing O-F bonds have been used for many years, only in recent years they found active application in the fluorination processes. Such reagents have attracted a great interest of chemists [8,10]. In spite of their usually high activity and moderate stability, they are found to be useful reagents in organic synthesis. More over, thanks to the work of prof. Rozen's team, new opportunities to use these compounds not only as fluorinating agents but also as unique oxidizers appeared; that made possible to carry out a number of important (from practical standpoint) processes to produce epoxides, to oxidize olefins etc. [10,17].
A recent synthesis of novel unique reagents of fluorooxytrifluoromethane, CF3OF, and cesium fluorooxysulfate, CsSO3OF, has been an event in this field that determined an increased interest of experts. Probably, for the first time researchers have on hand fluorooxy-reagents that could be isolated and used in individual state- in difference to majority of other fluorinating O-F reagents used as a rule in situ. These works initiate an extensive scope of investigations on the structure, reactivity and properties of compounds containing labile fluorine atoms at a heteroatom. They were aimed at development of a theory and methods of a synthesis of materials of new generation with attention to problems of technique, medicine and agriculture.
CF3OF and CsSO4F, which gave a powerful incentive to the development of researches, however have not been developed on an industrial scale due to a high cost and hence commercial impracticability of CF3OF and due to insufficient safety in handling of CsSO4F because of its ability to spontaneous at once decomposition (as a weak explosion, "clap") and is produced in small amounts only at the place of its use, in laboratories.
That is, in addition, one of the reasons that in daily practice the O-F-derivatives as fluorinating reagents yield to N-F-amines, stable compounds available in great variety and produced on an industrial scale.
In this review we have made an attempt to summarize and systemize new data on synthesis of fluorine organic compounds and a number of processes with the use of reagents containing O-F bonds. The main attention was paid to the comparative characteristics of these reagents, comparison of their opportunities with the fluorinating agents already in use and to reactions of the reagents containing O-F bonds with unsaturated compounds, heterocyclic and hetero-organic compounds. We do not examine OF2 as a reagent for the fluorination because it, the same as fluorine, is difficult and dangerous to store and it is not so available in addition. Moreover, different peroxides containing the O-F bond, for example CF3OOF etc., also were not included in our task.
Synthesis and characteristics of compounds containing O-F bonds.
Compounds containing the O-F functional group relate to the class of hypofluorites. Relative stability of these compounds depends on the nature of substituents at this group. Compounds with perfluoroalkyl (Rf) and perfluoroalkylcarbonyl substituents are stable and can be isolated as individual compounds at ordinary conditions. But it is not obligatory to isolate these compounds as individual ones; it is possible to use them in situ.
The fluorooxy or hypofluorite group (OF) has strong oxidizing and fluorinating properties. Thermodynamic stability of the compounds of ROF type is determined by the nature of R group. A direct bond with fluorine (OF2) or with perfluorinated fragment (CF3OF, SF5OF) leads to increasing stability whereas with unfluorinated fragments (CH3OF) results in instability.
Hypofluorites produced from inorganic and organic oxy-acids (NO3F, ClO4F,CF3COOF) were found to be unstable. At the same time acetylhypofluorite (CH3COOF) and hypofluorite acid (HOF) were produced and turned out to be stable enough for a long-term storage. Such solvents are used for carrying out fluorination of appropriate carboxylates at low temperatures.
The main method to synthesize hypofluorites is direct fluorination by elemental fluorine of proper predecessors. Thus, fluorooxytrifluoromethane was obtained by subjection of carbon monoxide to elemental fluorine in the presence of catalyst, CsF [18-21]. The reaction proceeds through intermediate formation of carbonyl fluoride and follows with a considerable heat release. CF3OF is stable in closed copper equipment up to 450oC and decomposes at 375oC.
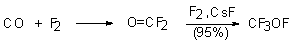
The subjection of carbon dioxide to elemental fluorine results in formation of bis-(fluorooxy)difluoromethane in 95% yield in a flow reactor [18-27]. In this case the high catalytic activity and selectivity of CsF is used. The process first is carried out at 77K and the temperature then is increased to room temperature, the yield of the goal product is very high.
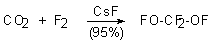
CF3OF is a gas with a specific odor of fluorine, it turns into liquid at –95oC. Difluoromethylenedihypofluorite has a boiling temperature of –64oC, decomposes at 150oC for some hours. Tetrafluoroethylenedihypofluoride CF3CF(OF)2 decomposes at 150oC for 65 hours only by 5%.
The fluorination of fluoroanhydrides of perfluorocarbonic acids results also in formation of appropriate hypofluorites. The reaction proceeds in the presence of fluorides of the following metals: Na, K,Pb,Cs,Mg,Ca,Sr,Ba,Fe, Cu, Ni, Ag and fluorides of CaF-HF, KAgF4 type.

Usually when sodium salt of trifluoroacetic acid is subjected to elemental fluorine, three hypofluorites are formed [28,29].
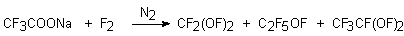
However, if the reaction is carried out in CFCl3 at –60oC, it is possible to obtain only trifluoroacetylhypofluorite [30]. This way was used to produce trifluoroacetylhypofluorite labeled by 18F tracer isotope.
When sodium salt of acetic acid in freon CFCl3 is subjected to elemental fluorine diluted with inert gas (10-15%) at –75oC, acetylhypofluorite is formed in high yield [31-35].
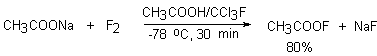
Ammonium salt [36] and potassium salt [37] of acetic acid at room temperature are also used. The properties of the reagents are given in papers [38-41]. But due to instability of CH3COOF it is possible to operate with it only at low temperatures. It is stable in polar solvents and begins to decompose at room temperature (t1/2 for 3 hours at 20oC). The investigation of the thermolysis of CH3COOF has shown that decomposition products are methyl fluoride and CO2 only [40]. In a system of AcONa-CHCl3/AcOH (10:1) at –60oC CH3COOF was obtained but only in low concentration. In paper [30] a system of MeCN/AcOH (10:1) as a solvent and fluorine diluted with nitrogen (15% concentration) were used at –45oC. In this case it was succeeded to obtain the reagent of a higher concentration.
Later Kadi synthesized trifluoroacetylhypofluorite, more stable in comparison with acetylhypofluorite, by reaction of elemental fluorine and trifluoroacetic acid in water. [42]. Better results were obtained in the reaction of fluorine and sodium salt of trifluoroacetic acid [33]
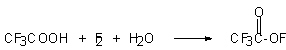
In case of perfluorinated carbonic acids, the reaction of their salts (Li,Na,K, Cs) with elemental fluorine is carried out in inert perfluorohydrocarbons, for example in perfluoro-2-butyltetrahydrofurane [43,44]. It should be noted that the salts of these acids (Ag, Ca,Cu) do not give appropriate hypofluorites [44].
The presence of electron-seeking groups at  -position determines hypofluorite stability [45].
-position determines hypofluorite stability [45].
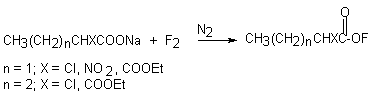
On the contrary, the presence of electron-donor CH3,Cl,H substituents , in the 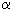 -position leads to a drastic decrease in stability.
-position leads to a drastic decrease in stability.
The reaction of elemental fluorine with alcohols results in formation of hypofluorites stable only at low temperatures, propionnitrile (for the synthesis of t-BuOF [46]) or acetonitrile ( for the synthesis of CH3OF [47]) are used for stabilization.
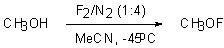
CH3OH is stable in liquid and solid state up to a temperature of –110oC whereas t-BuOF up to –40oC.
Reagent HOF MeCN was obtained when elemental fluorine (10-15%) diluted with nitrogen passed through an aqueous solution of acetonitrile under mild conditions, i.e. at 0-25oC for 3-4 hours [48].
Salts of fluorooxysulfates as a rule of alkaline metals are obtained by direct fluorination of the sulfates with elemental fluorine (20% in nitrogen) at 0oC in water for 5 hours (in 65% yield) [39,42].
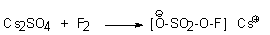
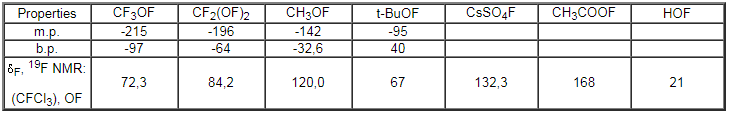
The standard oxidizing potential of SO4F- - HSO4- is 2.5 V [49]. Cesium fluorooxysulfate is a unique example of an ionic inorganic hypofluorite. This salt is convenient enough in handling. It is an excellent reagent for organic and inorganic synthesis and is widely used as a fluorinating agent. It was reported [50,51], that more than 200g of CsSO4F was produced and kept in a polyethylene bottle for 14 days at 0oC without obvious decrease in the fluorinating ability of the salt. Some characteristics of the reagents are given in Table 1.
Table 1.Physical properties of simplest compounds containing O-F bonds
Due to a relatively low energy of the bond rupture (O-F), the processes typically proceed according to a radical mechanism. Nevertheless, the reagent structure, in particular the structure of the reagent bound to oxygen, the process conditions (solvent ,catalyst, temperature) should amend. Thus, if a fragment bound with oxygen contains an electron-donor group, for example CH3, tret-Bu etc., then the O-F bond acquires an ionic character, and due to a greater electronegativeness of fluorine compared with oxygen, the fluorine of the O-F bond has the negative charge and the oxygen has the positive one. Because of the presence of the strong electron-seeking substituent at the oxygen that is able to stabilize the negative charge, polarization of the O-F bond will lead to a positive induced charge on the fluorine. But if the substituent (even it possesses a high inductive effect) is not able to stabilize the negative charge and the influence is executed according to an inductive mechanism (for example in the case of CF3 group), then such a compound has to show fluorinating properties that are realized according to the radical mechanism.The chemical properties of hypofluorites are determined by the nature of the O-F bond. The reactivity of this class of fluorinating agents strongly depends on the structure of the reagent and the organic substrate, on the reaction conditions( solvent, catalyst, temperature). The energy of the O-F bond little depends on the structure of fluorooxy-compounds and is within a range of 50-55 kcal/mol. Note that the energy of the N-F bond is 67-70 kcal mol. It follows from this comparison that the fluorine atom in the O-F reagents is bound less strongly. But the significance of this fact is not obvious in synthesis. High exothermicity of the reactions leads to a great number of side processes, sometimes the processes are accompanied by explosion.
Taken into account the abovementioned, it seemed reasonable to examine reactivity of the compounds containing the O-F bond from the standpoint of the O-F bond character.
Methyl- and tret-butylhypofluorites as electrophilic alkoxylating agents.
The name of the part itself says about peculiar chemical features of the two mentioned hypofluorites differed from all the abovementioned representatives of this class compounds.
In CH3OF and (CH3)3COF due to neighboring of the OF group with strong electron-donor partners, considerable rearrangement of the O-F bond state takes place in comparison with common hypofluorites where the OF fragment is bound to strong electron-seeking groups: in CH3OF and (CH3)3COF the positive charge is concentrated on the oxygen atom , and not on the fluorine atom, and, thus, the methoxy group, and not the fluorine, becomes the carrier of electrophilic properties of the reagent.
In accordance with that, CH3OF, which producing by Rosen's team became a recent sensation in the fluorine chemistry, is mainly a methoxylating (and not a fluorinating) reagent, namely CH3O+ group leads the reaction. CH3OF was studied more completely than (CH3)3COF. Successful generation of MeOF was observed in the reaction of olefins with XeF2/MeOH system [52]. But afterwards it turned out that the intermediate reagent and hence the reagent in this reaction was MeOXeF [53].
For the first time CH3OF was obtained in the reaction of elemental fluorine and a suspension of sodium methylate in CFCl3at –78oC [18,47]. The stabilizing effect of acetonitrile was then found and now the fluorination with elemental fluorine is carried out in a mixture of MeOH/MeCN. (CH3)3COF was produced similarly to CH33OF [46]. The structure of CH3OF was studied: the C-O-F angle was equal to 105oC, the O-F bond length was 1.45 A, the dissociation energy to radicals CH3O and F was 38 kcal/mol [54]. The thermolysis of this compound results in formation of hydrogen fluoride and formaldehyde. This is in conformance with the data of mass-spectrometric analysis of CH3OF molecule: the molecular peak m/e 50 (M+,70%) and 29 (HCO+, 100%) [47].
But attempts to produce EtOF, PrOF, CF3CH2OF, (CF3)2CHOF according to this method failed; hydrogen fluoride and products of alcohols decomposition were formed [55]. The use of completely deuterated alcohols, for example CD3CD2OD, has shown that DF is eliminated from the hypofluorite and CD3CDO is formed [55]. A similar result was obtained for CH3CD2OD as well.
Methylhypofluorite reacts with a number of typical olefins to give appropriate a-fluoroxy derivatives in 60-75% yield [47]. Thus, styrene and 1,1-diphenylethylene give 1-fluoro-1-phenyl-2-methoxyethane and 1,1-dephenyl-1-fluoro-2-methoxyethane respectively [56].
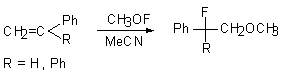
The addition of meta-dinitrobenzene does not influence the process. Obviously the reaction proceeds not through formation of radicals.
In CH3OF fluorine is not the electrophilic end of the molecule. It should be noted, that various electrophiles, for example “Cl+”, “F+”, “MeO+”, are fixed only in a transient state of the molecule at polarization and it is rather difficult to examine routes of the reactions with sources of such electrophilic particles ( see the discussion on the subject in paper [57]).
With nonterminal alkenes, for example trans- and cis-stilbenes, CH3OF gives threo- and erythro-1-fluoro-2-methoxy-1,2-diphenylethanes. Thus, preferably syn-addition takes place.
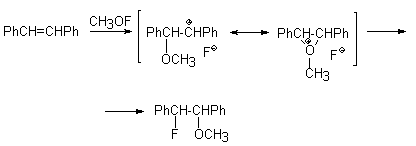
Acenaphthylene reacts with CH3OF to form trans- and cis-1-fluoro-2-methoxyacenaphthylene (2,1/1) and cyclooctene gives 1-fluoro-2-methoxycyclooctane [46].
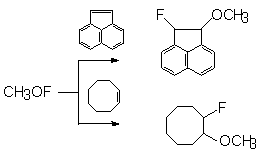
Trans-1-fluoro-2-methoxyindene was produced by CH3OF action on indene.
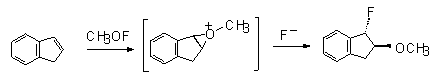
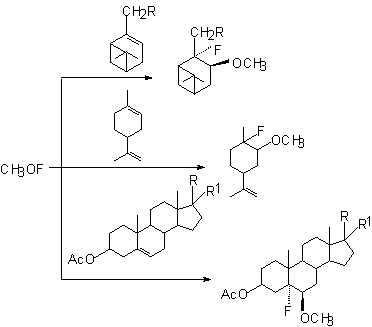
Polycyclic compounds were successfully involved into the reaction with CH3OF to form adducts to the double bond [58].
When 1-indanone reacts with CH3OF, 2-methoxy-1-indanone is formed in 70% yield.
In contrast to other hypofluorites, CH3OF in reactions with olefins containing substituents at the double bond gives not 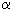 -fluoroketones but
-fluoroketones but 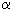 -methoxyketones.
-methoxyketones.
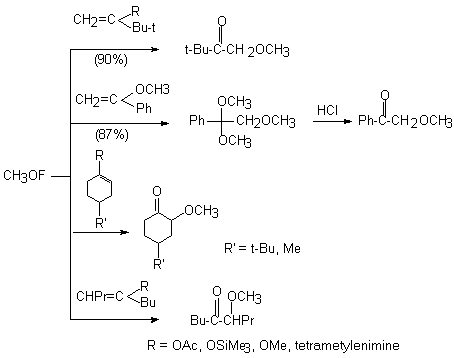
(CH3)3COF like CH3OF is an effective electrophile also. The interaction with olefins gives adducts to the double bond as the main reaction product, whereas 1-fluoro1-phenyl-3-cyanpropane is formed in acetone [46].
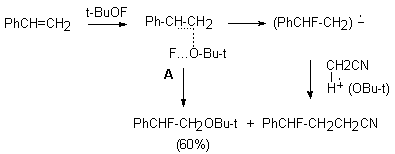
The reaction probably proceeds through transition state A that either is fluorinated to form the anion-radical or the reagent is added to the multiple bond. In this case (CH3)3CO group is found at the carbon atom ( carrying the largest negative charge) of the multiple bond. A similar reaction under analogues conditions takes place between tret-butylhypofluorite and 1,1-diphenylethylene as well, where 2 products are formed : 1-fluoro-1,1-diphenyl-2-tret-butoxyethane and 1-fluoro-1,1-diphenyl-3-cyanpropane.
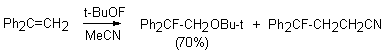
Meanwhile, even using this reagent in a considerable excess (7-10 moles), it is impossible to increase the conversion above 60%. Trans-1-phenyl-1-propene does not react with tret-BuOF to give erythro- and treo-1-fluoro-1-phenyl-2-tret-butoxypropane (in a ratio of 3:1)
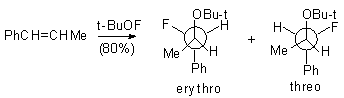
In case of naphthylene , probably due to stabilization of intermediate oxonium cation B, only threo-1-fluoro-2-tret-butoxyacenaphthylene is formed ( 60% conversion).
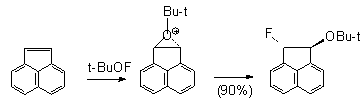
B
The reaction with styrene gives 1-fluoro-1-phenyl-2-tret-butoxyethane in 60% yield and with 1-vinylnaphthalene it gives 1-fluoro-1-naphtyl-2-tret-butoxyethane ( in 60% yield) [59].
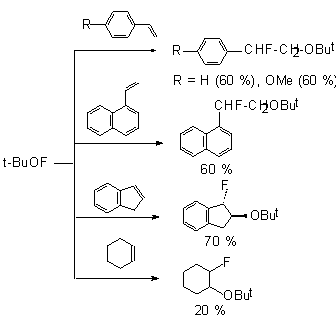
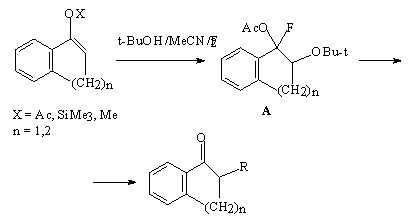
The reaction of tret-BuOF with enolacetate of 1-indone gives unstable compound A that is converted to 2-fluoro-1-indanone [59]. The tetralone derivative reacts similarly to give 2-fluoro-1-tetralone.
Tret-butoxylation of aliphatic and nonbenzene ketones under subjection to tret-butylhypofluorite may be represented as a new approach to produce such derivatives.
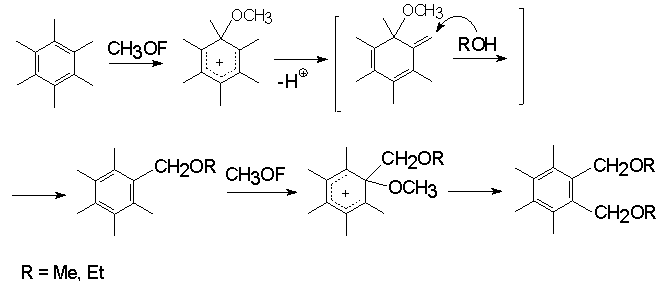
The interaction of CH3OF with aromatic derivatives gives methoxy-substituted ones [60]. Thus, mesytelene and durene at –45oC react with CH3OF to produce 2,4,6-trimethylanisole and 2,3,5,6-tetramethylanisole in 50% and 90% yield respectively.
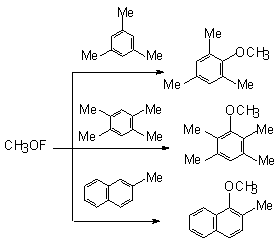
The electrophilic nature of the reagent has been confirmed by regioselective methoxylation of 2-methylnaphtaline where 1-methoxy-2-methylnaphtaline is formed in 30% yield.
Hexamethylbenzene reacts with CH3OF in alcohols to give mono- and dibenzylmethoxylated products of substitution in 40 and 25% yields respectively [60].
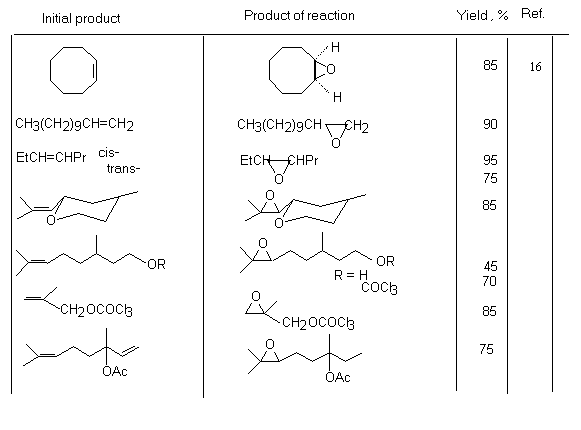
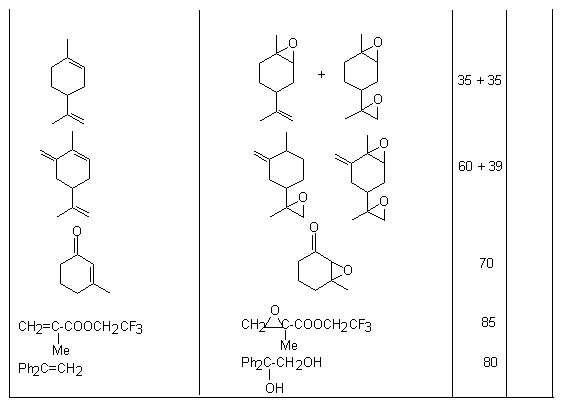
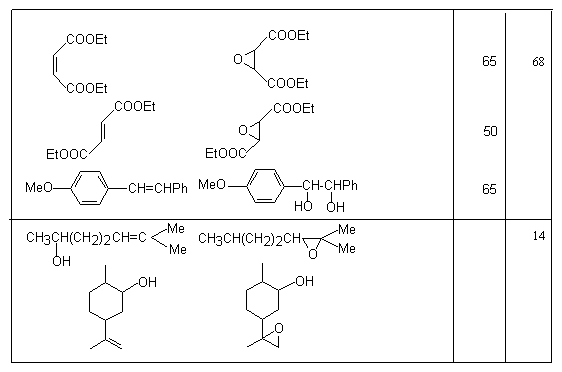
Application of HOF*MeCN reagent as an oxidizer of unsaturated compounds.The formation of this route is bound with Appelman name who synthesized HOF in 1973[61], studied its structure [62,63], 19F NMR spectrum and made X-ray analysis of HOF crystals [65,66]. The first investigations of chemical properties of HOF have already shown that this reagent is able not only to be added to olefins to the double bond but also possesses the high oxidizing ability [67]. In this respect it is similar to F2O [68]. Thus, interaction of HOF with sulfides, naphthaline and derivatives of acetylene gives oxidation products [14,69-72].
Meanwhile, this hypofluoric acid (HOF) was well-known earlier [73]. But it was not used in organic chemistry because of its excessive instability. However in recent years a new approach was developed in production and stabilizing this compound. It happened due to the studies by S.Rozen who has developed the new approach in stabilization of this compound by means of the formation a complex of HOF with acetonitrile HOF MeCN [17,70,71]. This work exerted stimulant influence on the process of formation of reagents containing the O-F bonds and on development of processes of fluorination and oxidation of organic molecules. The high oxidizing properties of HOF discovered a new synthetic approach to the possibility to oxidize unsaturated compounds in a simple and extremely effective way. The X-ray analysis of this complex has shown that the H-O-F angle is 90oC and the length of the hydrogen bond between the nitrogen atom and hydrogen is 1.7A [74]. This complex is considerably more stable than HOF and its solution of the concentration up to 1 mole can be produced (for the HOF solution only concentration of ~ 1mmole is permissible at room temperature). The entropy of this complex formation is –14.3kJ [75]. These studies have discovered new synthetic approaches to use HOF as an excellent agent for oxygen “transportation”. Its ability to execute a direct epoxidation of a multiple bond, hydroxylation of the inert C-H bond at tertiary carbon atom and oxidation of aromatic compounds[30], dimethyl dioxirane [42] , fluorine-containing olefins [78], sulfides [77], conversions of aromatic and aliphatic amines [78,79] and alcohols [80,81] to the appropriate nitro-derivatives and ketones , conversions of ethers into aldehydes and acids has already been shown. Appearance of fluorine-containing compounds able to transform organic molecules into compounds without fluorine atoms shows one of remarkable properties of fluorine containing compounds that influenced much development of methods of synthetic organic chemistry. The determination of conditions of epoxidation of olefins is among new methods. Taken into account that HOF is made by passing elemental fluorine through an aqueous solution of acetonitrile, the developed method essentially is a smart and effective way to use fluorine for oxidation of olefins. Thus, as it was shown in [17], passing elemental fluorine through an aqueous solution of acetonitrile forms an oxidizer that converts trans-stilbene to f trans-stilbene oxide in 90% yield for 1 minute at 0oC. (E)-stilbene gives a mixture of benzophenone and threo-2-fluoro-1,2-diphenylethanol [69].
Traditional oxidation of olefins under subjection to H2O2/MeCN proceeds slowly and requires elevated temperatures. But in this case the system of F2/H2O2/MeCN reacts almost immediately at 0oC. The examples of epoxidation reactions are given in table.2. Table 2. Epoxidation of unsaturated compounds under the influence of HOF-MeCN reagent
The high electrophilicity of this reagent, the reaction rate and complete configuration transformation point to the two-stage process that includes
B If the stable
When water is used with the oxygen isotopes ( H2O 18, H2O17) 97% of isotopic mark is found in the epoxy cycle [17].
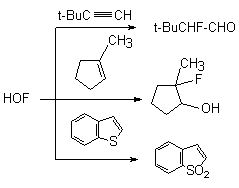
Fluorosubstituted olefins under subjection to this system give epoxides in good yields [76].
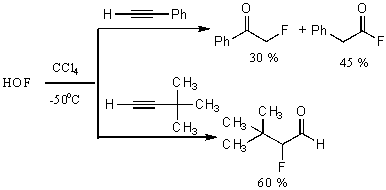
When this reagent reacts with phenylacetylene , 2-fluoro-1-phenylethanone and phenylacetylfluoride are formed [67].
If tret-butyl substituent is at the triple bond(for example, for 3,3-dimethyl –1-ine) then 2-fluoro-3,3 dimethylbutanal is formed. Oxyethylporphyrin is converted to N-oxide of octaethylpophyrin [82]. The hydrogen atoms at the tertiary carbon atoms are successfully hydroxylated [83]. This approach is used to introduce 18O isotope into molecules.
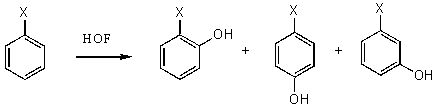
Hypofluoric acid (HOF) in a reaction with some aromatic compounds gives phenol derivatives and an increase in orto-isomers content draws attention [17].
The reaction with naphthalene proceeds extremely slowly to give 1-oxynaphthol in 2.9% yield merely, 2-oxynaphthol in 0.75% yield and 1,4-naphthoquinone in 7.4% yield [69]. In the interaction of HOF-MeCN complex with aromatic and polycyclic compounds the latter are oxidized to quinones ( table 2)[60].
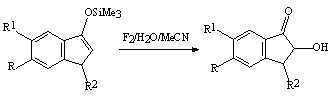
Oxidation of compounds containing a carbonyl group proceeds more readily. Thus, a solution of HOF-MeCN complex interacts with trimethylsilyl ether of 1-indanone enol to give 2 hydroxy-1-indanone at 0oC for 3-4 minutes [84].
R=R1=R2= H 88 % R=R1= OMe, R2= H 92 % R= OMe, R1=R2= H 93 % R=R1= H, R2= Me 98 % R=R1= áåíçî, R2= H 92 % HOF-MeCN complex currently is the best reagent in organic synthesis for oxygen transportation and effective oxidation of unsaturated organic compounds. It is environmentally appropriate oxidizing reagent. If the benzene ring contains a group certainly inclined to oxidation then the process proceeds under especially mild conditions. For example, when this oxidizers affects anilines, the appropriate nitrobenzenes are formed [78,79].
It should be noted that 4-benzaminic acid and 3-aminophenol give the appropriate nitro-derivatives also, while perbenzoic acid and quinone are not formed in this case in contrast to oxidizers based on peroxides. Aliphatic amines are oxidized to the appropriate nitro-derivatives as well. 3-Aminomethyl-3,5,5-trimethylcyclohexanol converts to the nitro-derivative without involving the OH-group [80].
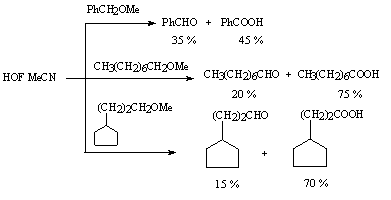
HOF-MeCN complex is used for oxidation of secondary alcohols and ketones according to the Baeyer-Villiger reaction [80]. Some ethers containing the methyl group are subjected to oxidation also (Table 2) [85,86].In this case ketones, there are formed aldehydes and acids.
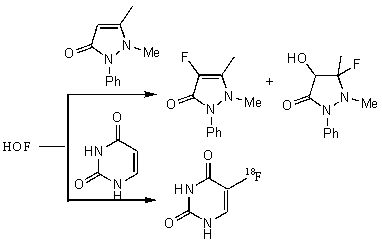
Methyl(trans-4-tert-butylcyclohexyl) ether (ester?) under action of HOF MeCN is converted to 4-tert-butylcyclohexanone in 90% yield under mild conditions [81]. It should be noted that in the reaction of oxidation of ketones with this reagent in a solution of acetonitrile the formation of a-oxy ketones is observed. Antipyrin gives 3- and 4-fluoro-derivatives whereas uracil gives 5-fluorouracil under subjection to HOF produced by fluorine interaction in situ [87].
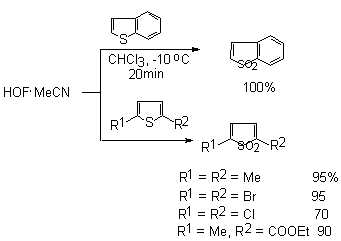
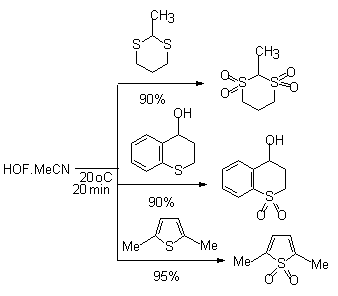
In sulfur-containing heterocyclic compounds containing 2-valence sulfur atom the latter is oxidized to 6-valence one [42]. Thus, benzothiophene and 2,5-substituents of thiophene under the influence of HOF-MeCN give 1,1-dioxides [69].
Six-membered sulfur-containing heterocycles behave similarly.
If 2,5-dibromothiophene with HOF-MeCN gives S,S-dioxide 2,5 dibromothiophene in 95% yield, whereas dimethyl dioxirane is oxidized in 27% yield as maximum. Sulfides containing aryl or perfluoroalkyl groups under the influence of this complex are effectively oxidized to the appropriate sulfones [88,89].
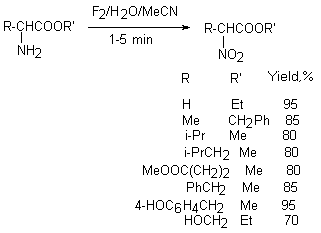
Polyfluorovinyl ethers containing a SR group, for example CF2=CFOCH2CF2CF2SR, are oxidized with replacement of the sulfur atom using HOF-MeCN complex to form CF2SO2H groups that allows producing after polymerization polymers used as membranes and durable films [90]. Amino acids ( ethers of ethylglycine, alanine, valine, leucine etc.) under action of HOF-MeCN are converted to nitro-derivatives [91].
|
Continuation in the next volume
Fluorine Notes, 2001, 16, 1-2
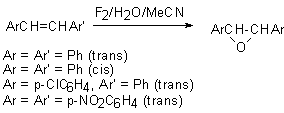
 -oxycarbcation B
-oxycarbcation B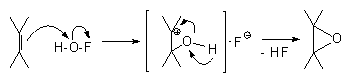
 -oxycarbcation is formed, transformation of the alkene to vicinal glycol is possible. Thus, in the reaction of HOF-MeCN with 1,1-diphenylethylene and 4-methoxystilbene at –15oC the appropriate glycols are formed, for example 1,1-diphenylethane-1,2-diol.
-oxycarbcation is formed, transformation of the alkene to vicinal glycol is possible. Thus, in the reaction of HOF-MeCN with 1,1-diphenylethylene and 4-methoxystilbene at –15oC the appropriate glycols are formed, for example 1,1-diphenylethane-1,2-diol.