Fluorine Notes, 2001, 14, 9-10
Synthesis of n-polyethoxyamides of perfluoroaliphatic acidsS.S.Gonek, D.Ya.Kasaeva, K.I.Serushkin Production of stable emulsions of perfluoroorganic compounds, oxygen carriers, being the basis for synthetic blood is impossible without using effective non-toxic emulsifiers. A range of surfactants currently used is very limited due to extremely strict requirements to these compounds. The most perspective to these purposes might be fluorine-containing surfactants of non-ionogenic type possessing high surface activity and biological inertness. The purpose of this study is development of a method to produce n-polyethoxyamides of perfluoroalkylcarbonic acids and perfluoroalkanesulfo acids, highly effective non-ionogenic fluorosurfactants of the general formula: FZNR(CH2CH2O)mH , where Rf=perfluoroalkyl, perfluorooxaalkyl; Z=CO,SO2; R=H,C4H9; m=2 - 30. There is known a method to produce oxyethylated amides of polyoxaalkanecarbonic acids with m =10 including interaction of appropriate amidoethanols with ethylene oxide under pressure at 90oC in the presence of catalyst, boron trifluoride etherate [1-3]. A disadvantage of this method is a necessity of using special equipment (an autoclave) and expensive catalyst. It was reported [1-3] about a method to obtain a surfactant of the mentioned structure (Rf=C6F13, Z=CO, m=2-6) by interaction of polyethoxylated amines with perfluoroenanthic acid in the presence of a complex catalyst according to the scheme: C6F13COOH + NH2(CH2CH2O)nH
But this method is difficult of access because of difficulties to obtain polyethoxylated amides of perfluorocarbonic acids from appropriate esters according to the scheme: CnF2n+1COOC2H5 + NH2CH2CH2OH
, where n=4,6,8. Synthesis of intermediate ethanolamides was carried out at a molar ratio of the reagents of ethyl ether : monoethanol amine=1:1,2 ; the reaction product was purified by water washing or by crystallization from chloroform. Purified ethanol amides were crystal substances of white colour with m.p. from 46 to 93oC. The process of ethoxylation was carried out by bubbling gaseous ethylene oxide at atmospheric pressure through the melt or through a solution of the intermediate ethanolamide in the presence of catalyst. Triethylamine was used as the catalyst. It has been found that the using of acid catalysts is not effective, in the presence of alkaline catalysts (concentrated solutions of NaOH, KOH) hydrolysis of the amide bond of ethanolamides takes place. The influence of the catalyst concentration on the rate of the process and yield of the target product has been studied. It was found that the ratio of ethanol amide to triethylamide of 1:0,4 was optimal. An increase in the catalyst concentration resulted in resinification of the reaction mass. The choice of the optimal temperature was caused by the melting temperature of ethanolamide on the one hand and on the other hand by the fact that at a temperature above 95oC the process was followed by destruction of the carbonyl group of ethanolamide to form fluorocarbons of CnF2n+1 type with b.p. 32oC. A temperature of 70-80oC is optimal for ethanolamides of perfluoroenanthic and perfluorovaleric acids. The oxyethylation of ethanolamide of perfluoropelargonic acid (m.p. 93oC) was carried out in a solution of fluorocarbons at a temperature of 80-85oC. In this case the oxyethylation products (m>2) were not dissolved in fluorocarbons and could be removed from the reaction zone without decomposition. The produced n-polyethoxyamides of perfluorocarbonic acids with a various degree of oxyethylation are viscous transparent oils of a straw to red-brown colour. Aqueous solutions of these compounds have a low surface tension (~ 2-mN/m at c<1%) and form stable emulsions with perfluorodecaline, R-113 and other fluorocarbon liquids. The average statistical degree of oxyethylation "m" was determined by the increase in weight of the reaction mass and defined more exactly by PMR spectrometry method by comparison of the integral intensity of the joint spectral line of oxyethyl groups in the end compound with the integral intensity of the line corresponding to the three groups C2H5 of triethylamine used as the catalyst with a strongly fixed concentration. Oxyethylated butylamides of perfluorooxaalkanesulfo acids were obtained by a known method [1-3] including bubbling gaseous ethylene oxide at 90-100oC into a mixture of initial butylsulfonamido alcohol and a catalyst, 50% solution of caustic soda.
The numerical value of "m"was determined according to the increase in weight and specified by a method of PMR spectroscopy comparing the integral intensities of the oxyethyl groups with the integral lines corresponding to C4H9 group. Experimental. 1. Monoethanolamide of perfluoroenanthic acid 100.0 g (0.25 mole) of ethylate of perfluoroenanthic acid was charged into a reaction flask equipped with a stirrer, backflow condenser, dropping funnel, thermometer. 18.3 g (0.3 mole) of monoethanolamine was added at vigorous stirring with such a rate that the reaction mass temperature would not exceed 75oC. After completion of adding ethanolamine, the reaction mass was cooled, washed with 200 mL of water, the product was filtered through a Buchner funnel and dried in vacuum. Thus, monoethanolamide of perfluoroenanthic acid, a solid crystal substance of white colour, was produced. The yield was 96.8g (94%), m.p.70oC. Ethanolamides of perfluorovaleric and perfluoropelargonic acids were obtained in a similar way, m.p. 46 and 93 oC respectively. 2. N-polyethoxyamide of perfluoroenanthic acid. 40.0g (0.09 mole) of ethanolamide of perfluoroenanthic acid and 3.9 g (0.039 mole) of triethanolamine were charged into a glass cylindrical reactor equipped with a stirrer, thermometer, bubbler, backflow condenser joint to a control bottle filled with water. The reaction mass was heated at vigorous stirring up to 70oC and ethylene oxide was added with such a rate that there would not be any bubbles in the control bottle. The consumption of ethylene oxide was determined with a rheometer. To remove triethylamine and volatile admixtures, the product was aged at 100oC under vacuum. The product then was replaced in a flask equipped with a backflow condenser, stirrer, thermometer, dissolved in 300 mL of acetone and the solution was boiled with activated carbon for 2 hours, it then was cooled, filtered and dried at 100oC under vacuum. The product produced was a mixture of polyethoxyethanolamides of general formula C6F13CONH(CH2CH2O)mH, where m=15.56, a viscous oil of a red-brown colour. 3.N-Polyethoxy-N-butylperfluoro(4-methyl-3,6-dioxaoctane)sulfonamide. 58.5 g (0.10 mole) of N-butyl-Nethanolperfluoro(4-methyl-3,6-dioxaoctane)sulfonamide and 2.4g of 50% solution of NaOH were charged into a reactor equipped with a stirrer, backflow condenser, thermometer and bubbler. The reaction mixture was heated at vigorous stirring up to 100oC and ethylene oxide was charged with a such rate that overshoot of bubbles in the control bottle was absent. The product of oxyethylation was aged at 100oC for 1 hour and cooled. The reactor then was charged with 300 ml of acetone, the solution was boiled with activated carbon for 2 hours. The solution then was cooled, filtered, the solvent was distilled and the dry residue was dried at 100oC under vacuum. Thus a mixture of polyoxaethyleneamides of general formula
was produced, a viscous oil of a straw colour. References 1. Novoe v tekhnologii soedinenij ftora / Pod red. N. Isikawa. - M: Mir. 1984. - S. 385-403. 2. Soedineniya ftora. Sintez i primenenie / Pod red. N. Isikawa. - M: Mir. 1990. - S. 157-182. 3. Ftor. Khimiya i primenenie / Pod red. N. Isikawa‚ Y. Kabayasi. - M: Mir, 1982. - S. 210-220. |
Fluorine Notes, 2001, 14, 9-10
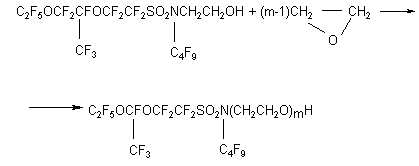
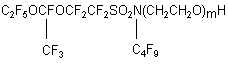 , where m=8.1
, where m=8.1