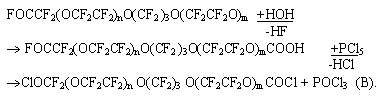Fluorine Notes, 2001, 14, 7-8
Application of perfluoroacylfluorides in the synthesis of perfluoroalkylvinyl ethers IV.Synthesis of bifunctional oligomers of tetrafluoroethylene oxide. A.E.Ershov, L.M. Popova Frost resistance increase is an important problem in chemistry of fluoropolymers. One of the ways of solving this problem is introduction of oxygen atoms into fluoropolymers that allows reducing significantly the glass transition temperature of these thermally stable and chemically inert compounds. The methods of synthesis of monomers for creation of fluoropolymers with a reduced glass transition temperature [1,2] and the dependence of the frost resistance of the fluoropolymers on the structure of polyfluoroalkylvinyl ethers was discussed in previous papers [3]. This paper gives a method to synthesize bifunctional oligomers of tetrafluoroethylene oxide from which polyperoxides were produced used in the process of radical copolymerization of the monomers as initiators and influenced on the glass transition temperature of the polymer formed. To produce bifunctional oligomers of tetrafluoroethylene oxide (TFEO), a rotary absorber was charged with a solution of potassium alkoxide KOCF2CF2COF in diglyme which was preliminarily obtained by the reaction of perfluoromalonyl fluoride with potassium fluoride :
The rotary absorber was joined to a reactor of photo-chemical oxidation of tetrafluoroethylene (TFE) and at a temperature of -10 -30oC a mixture of oligomeric products was obtained: FOCCF2(OCF2CF2)nO(CF2 )3O(CF2CF2O)mCF2COFm + n = 0,1,2,3... , (A) (85- 90%) CF3O(CF2CF2O)nCF2COF n = 0,1,2... , (В) (10- 15%). On the basis of diacylfluorides (A) a number of diacylchlorides was synthesized:
On the basis of diacylchlorides (B) and diacylfluorides (A) there were obtained appropriate polyperoxides of the following general formula: -[-ООCCF2(OCF2CF2)nO(CF2)3O(CF2CF2O)mCF2CОО-]-x , where n+m=2-5 is the degree of polymerization by a reaction of diacylchlorides or diacylfluorides with an aqueous solution of sodium peroxide in a medium of R-113 at -6 - +6oC. The peroxides obtained were used as the sources of free radicals in the form of 15% solutions in R-113 in the process of co-polymerization of vinylidene fluoride with perfluoromethylvinyl ether. As a consequence of introduction of the oxygen-containing fragments of the peroxide (5-6% mole) into the main chain of the co-polymer, all synthesized copolymers have the glass transition temperature by 10-15oC lower than copolymers of vinylidene fluoride with perfluoromethylvinyl ether. Obviously, for further reduction of the glass-transition temperature it is necessary to introduce a larger amount of the oxygen-containing fragments of the peroxide into the block-copolymer. Experimental Synthesis of diacylchloride B with n+m=3 of the following formula: ClOCF2(OCF2CF2)nO(CF2)3O(CF2CF2O)mCF2 COCl A flask was charged with 137 g of a mixture of dimethyl ethers of dicarbonic acids of the general formula: MeOOCF2(OCF2CF2)nO(CF2)3O(CF2CF2O)mCF3COOMe with n+m=3 and 25 mL of 85% formic acid was added and heated at 160oC. The formic acid was distilled and 124g of dicarbonic acid was discharged. The yield was 94%. The acids with n+m=4 and n+m=5 were obtained in a similar way. Then 85g of PCl5 was charged into a flask and 87g of dicarbonic acid with n+m=3 was added dropwise. Termination of hydrogen chloride evolution was an evidence of the reaction completion. After distillation of POCl3 and vacuum rectification there was produced 85g of diacyl chloride B with n+m=3. (b.p.82oC/1 Torr) (table). The yield was 96.6%. The diacylchlorides with n+m=4 and n+m=5 were obtained similarly, the analytical data are presented in the Table. Table
References
|
Fluorine Notes, 2001, 14, 7-8