Synthesis and application of 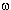 -bromo-perfluoroalkylvinyl ethers. -bromo-perfluoroalkylvinyl ethers.
V.M. Andrushin, N.B.Pavlova
Solution of more and more complex tasks in different fields requires development
of new structural materials possessing a fundamentally different complex
of field performances. Analysis of scientific and technical literature has
shown that studies based on application of perfluoroalkylvinyl ethers (PFAVE)
are the most priority directions in the field of development of new polymeric
materials.
This is confirmed, in particular, by intensity of researches in synthesis of
PFAVE monomers, in development of optimal conditions of copolymerization
of PFAVE with different fluoroolefins, in studies of structure, properties
and conditions of processing co-polymers produced and by more and more expanding
field of their application.
Fluoropolymers containing functional groups are of a particular interest. They
retain all positive features of fluoroplasts and at the same time exceed
them with regard to a number of field performances such as hydrophilicity,
electroconductivity, solubility, processibility, anti adhesion properties
etc.
We think it reasonable in this final report on 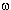 -bromo-perfluoroalkylvinyl ethers (BrAVE)
to review main conditions and fields of PFAVE application as a whole and
also a possible role of BrAVE in development of new fluoropolymers on the
basis of PFAVE monomers. -bromo-perfluoroalkylvinyl ethers (BrAVE)
to review main conditions and fields of PFAVE application as a whole and
also a possible role of BrAVE in development of new fluoropolymers on the
basis of PFAVE monomers.
Japanese firms ("Daikin", "Asahi Glass", "Nitto Danki Codio Co.Ltd", "Mitsui"
etc) "Du Pont" of USA and "Hoechst", Germany take their leading place in
scientific, production and commercial activities in problems of synthesis,
chemistry and application of PFAVE.
The work on development and study of new polymers derived from PFAVE are carrying
out according to the following directions:
- carboxyl-containing vinyl ethers containing functional groups COORf,
where Rf=H; alkyl and perfluoroalkyl C1-C10;
alkaline metal or ammonium ion;
- sulfofluoride vinyl ethers containing functional groups SO2Rf where Rf=F; OH; OMe where Me= alkaline metal or ammonium ion;
alkyl and perfluoroalkyl C1-C10; aryl C1-C10;
NHR1 where R1 is an alkyl;
- perfluorovinyl ethers containing the end CF2R group where R is a haloid or
hydrogen;
- perfluorovinyl ethers containing several functional groups, for example,
two carboxyls;
- Perfluorovinyl ethers containing a ketone group or ionogenic groups of phosphonic
acids; rather exotic methods of their synthesis do not allow to forecast
their practical application and they will not be under review further.
The study of conditions of co-polymerization of PFAVE with tetrafluoroethylene
(TFE) has shown that to obtain co-polymers with a significant number of PFAVE
chains , their considerable excess regarding TFE is required. Thus, the co-polymerization
constant for carboxyl PFAVE is 0.14 and 7.0 for TFE. In a reaction with sulfofluoride
PFAVE the co-polymerization constants are even lower: 0.8 and 8.0 for TFE.
A careful choice of medium conditions, a co-polymerization method, initiator,
pH, temperature, process regulators etc. is necessary in the synthesis of
co-polymers derived from TFE and PFAVE. It is necessary to take into account
that a phenomenon of fragmentation of the radical of the end chain of an
ether monomer takes place in the co-polymerization process according to the
scheme:
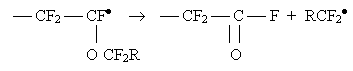
This fragmentation reduces considerably the molecular mass of the co-polymer.
The fragmentation is increased with the temperature and with the increase
in the length of a perfluoroalkoxyl substituent , therefore the process is
recommended to carry out at the possibly lowest temperature when the activation
energy of the fragmentation reaction is great.
The main methods of the co-polymerization of PFAVE with fluoroolefins is an emulsion
method and co-polymerization in organic solvents. The emulsion method is
preferable, it provides a more high conversion of PFAVE. Besides, the use
of solvents is fraught with transfer the chain to the solvent. As a whole,
the emulsion co-polymerization provides better reproducibility of the results
and production of co-polymers with a greater molecular mass.
But the emulsion polymerization requires a more high level of technology and
strong adherence to parameters, particularly to pH of the medium with the
purpose to avoid hydrolysis of ether groups, particularly in carboxyl PFAVE.
Mechanical properties of functional perfluorinated polymers depends greatly on
the type and PFAVE content. So, for example, tensile strength of carboxyl-containing
polymers as sodium salts is much higher than those in the form of methyl
ethers and the difference becomes more considerable with the temperature
growth ( it is 320 and 250 kg/cm2 at 25oC and 230 and
7 kg/cm2 at 90oC respectively). Relative elongation
of tapes and samples containing ether groups is essentially higher than that
of salts.
The glass transition temperature increases considerably at transition from ether
groups to acid groups and reduces with an increase of the content of PFAVE
chains.
As regards flowability of co-polymers of PFAVE with TFE, it depends on probability
to form intermolecular bonds, for example, hydrogen bonds in co-polymers
containing carboxide groups, and on cross-linkage .
In literature BRAVE monomers are presented rather stingy and the main task of
this series of reports on the synthesis, properties and application of this
class of fluoromonomers is to attract attention of researchers, experts and
managers to implementation of high potential capabilities of BRAVE both for
creation of new materials and for modification of the existing types of fluoromonomers.
It was shown in reports 1-6 published earlier, that BrAVE monomers could be synthesized
from available raw materials without using exotic experimental methods in
specialized research centres possessing sufficient practice in handling fluorine
and its compounds, also some data on the conditions of co-polymerization
of fluoroolefins with BrAVE, main properties of the fluoropolymers produced
and some conditions of their processing were given.
A specific feature of BrAVE monomers is the presence in their structure of the
perfluoroalkylene fragment, trifluorovinyloxide group and highly reactive
bromine atom in the w-position at the unsaturated bond. Their combination
allows producing fluoropolymers possessing a complex of positive service
properties peculiar to PFAVE co-polymers and functional activity as well.
Namely the combination of the mentioned properties allows to consider BrAVE
monomers in future as a base fluoromonomer together with tetrafluoroethylene,
vinylidene fluoride, hexafluoropropylene etc.
Demand in functional fluoropolymers containing carboxyl, sulfate, sulfofluoride,
amine, nitrile and other groups in dependence on the task under consideration
is currently rather low . Therefore an idea to establish a production of
BrAVE monomers and producing from them appropriate monomers with necessary
functional groups can be unprofitable from economic and technical point of
view. No doubt that drastic development of any route will make the original
synthesis of the appropriate target PFAVE and co-polymers derived from it
more expedient but these advantages can be achieved ,as a rule, in a large-scale
specialized production that is unlikely in the foreseeable future.
The most interesting and perspective route of PFAVE application is their using
in producing ion-exchange materials for membrane technology.
Development of the membrane technology in production of chlorine and caustic
soda on an industrial scale allows to solve ecological problems ( to exclude
using mercury and asbestos), to obtain products of a higher quality at lower
power expenses. These technologies are used most broadly in Japan, Netherlands,
the USA, Germany on the basis of perfluorinated ion-exchange membranes of
Japanese and American made.
Long before chlorine-alkaline electrolysis , membranes on the basis of PFAVE
with sulfo groups, possessing strong oxidizing properties and providing high
mobility of hydrogen, have been used as solid electrolytes in hydrogen-oxygen
and hydrogen-haloid fuel elements.
Application of fluorinated membranes able to provide high current density and
process effectiveness in water electrolysis is perspective in hydrogen power
engineering and in processes of separation of and drying gases. Perfluorinated
sulfocationite membranes can be used as ion-selective electrodes.
The following monomers are typical representatives of this group :
CF2=CF-O-CF2CF(CF3)OCF2CF2-SO2F
(Du Pont)
CF2=CF-O-[CF2CF(CF3)O]n (CF2)m AR (Asahi Kasei) where n=0.1; m=3-5; A=S; SO2; R=aryl,alkyl, perfluoroalkyl
C1-C10
CF2 = CF - O -CF2CF(CF3)OCF2CF2 - COOCH3 (Du Pont)
CF2=CF-O-CF2CF(CF3)O (CF2)3-COOCH3 (Asahi Garasu)
CF2=CF-O-(CF2) 2-4-COOCH3 (Asahi
Glass)
The latter monomer can be readily produced from BrAVE-2 monomer according to
the scheme:
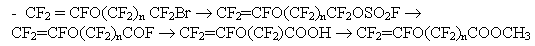
We have tested this method both in the synthesis of the monomer with 99.9% purity
and in processing tapes and granules of co-polymers of TFE with BrAVE-2.
The latter method of polymer-analogous transformations is of a particular
interest,because the constants of BrAVE co-polymerization exceed greatly
the appropriate characteristics of both sulfofluoride and carboxyl PFAVE.
The co-polymerization with TFE rather readily gives fluoropolymers with BrAVe
content up to 26%.
Researchers pay rapt attention to development of an available method to synthesize
w-fluorosulfurylperfluoroethylvinyl ether which is urgently necessary for
development of membrane technologies and which can not be obtained according
to a conventional scheme of addition of hexafluoropropylene oxide to the
appropriate fluoroanhydride followed by pyrolysis of salts of the acid produced.
The preliminary experiments made by us give hope for successful bromine replacement
in BrAVE monomers with the sufofluoride group.
The method of producing anion-active fluoropolymers derived from TFE and BrAVE
has been described in detail in report 2 of the present series. The most
perspective synthetic route by the method of polymer-analogous transformations
was proposed, the samples of the membranes were made and they showed high
effectiveness in electrolytic processes.
To produce perfluoroalkylenedivinyl ethers from BrAVE, it is possible to use
conventional reactions of debromination:
CF2 = CFO(CF2)Br  CF2 = CFO(CF2)2nOCF
= CF2 CF2 = CFO(CF2)2nOCF
= CF2
Producing PFAVE from BrAVE is rather easy technically according to the following
scheme:
CF2 = CFO(CF2)nCF2 Br  CF2 = CFO(CF2)nCF3 CF2 = CFO(CF2)nCF3
The given list of BrAVE application in processes of creation of fluoropolymers
does not bear an exhaustive character, it only demonstrates a possibility
to use BrAVE in the synthesis of fluoroplasts with different functional groups.
One more extremely important way of BrAVE application is their modification with
participation of different fluoropolymers, because bromine atoms are convenient
active centres for formation of space-linked structures. Report 4 describes
the experimental results of cross-linking BrAVE fluoro-co-polymers and perfluorodivinyl
ethers with TFE and vinylidene fluoride using hexafluorodiphenylolpropane
and triallylisocyanurate as cross-linking agents. The physical and mechanical
properties of polymers of TFE and VDF with BrAVE and conditions of their
processing were given in reports 5 and 6. It was determined by experiments
that BrAVE monomers were highly effective modifiers of fluoropolymers. The
co-polymers on their basis are stable both in acid and in alkaline media.
They possess higher physical and mechanical properties and that relates equally
both to TFE and to vinylidene fluoride and the polymers produced can be processed
by conventional methods.
This report considers only BrAVE monomers with the linear carbochain perfluoroalkylene
fragment though monomers with a different structure of the fluoroalkylene
chain behave similarly in reactions of co-polymerization.
In conclusion, the authors thank all those who participated in different stages
of the studies, analyses and testing the materials produced.
If we managed to attract attention of some researchers to the subject under consideration
and make them to study the subject more attentively and profoundly in the
original papers, we think our task has been fulfilled.
|