Fluorine Notes, 2000, 13, 3-4
Received september 2000
Synthesis of partially fluorinated organic compounds with the use of perfluoro-2-methyl-2-pentene and phenol derivatives.Ki-Whan Chi, Hyun-Ah Kim, Eduard L. Zhuzhgov 1 and G.G. Furin 2Department of Chemistry, University of Ulsan, Ulsan 680-749, Korea 1State Universiry of Novosibirsk, 630090, Novosibirsk, Russia 2 Institute of Organic Chemistry, 630090, Novosibirsk, Russia
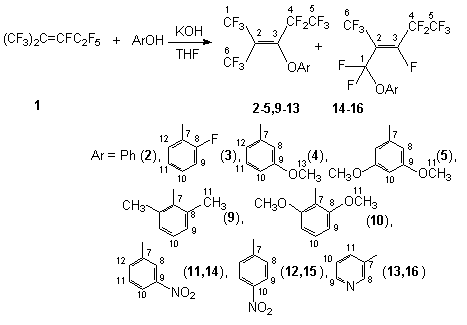
Introduction of fluorine atoms into organic molecules has brought to generation of series of new specific properties that are widely used in creation of up-to-date materials [1,2]. That considerably stimulates intensive development of the chemistry of fluorine-containing organic compounds that was caused by demands of technique and modern medicine. The class of bioactive compounds has been of growing interest after discovery of biological activity growth when fluorine atoms and perfluoroalkyl groups are introduced into bioactive compounds. Fluorine-containing intermediate products has been widely used for creation of both medicines and bioactive substances for agriculture [3,4]. Fluorine-containing diphenyl ethers and phenoxy-containing compounds take an important place among commercially used herbicides. One of approaches to synthesize compounds of this class is based on reactions of nucleophilic replacement of fluorine atoms with O-nucleophilic reagents in internal perfluoroolefins. Thus, it was shown that interaction of dimer and trimer of hexafluoropropylene in the presence of triethylamine [5,6] or sodium phenolate [7] results in formation of products of replacement of fluorine atoms at the double bond. Majority of aromatic compounds containing the OH group under conditions of catalysis with bases is effective O-nucleophilic reagents. The reactions resulting in the products of replacement of fluorine atoms at the internal double bond were described: between paracreosol and dimer or trimer of hexafluoropropylene [8], N-(4-hydroxyphenyl)acetamine and dimer of hexafluoropropylene [9], 3-oxybenzodioxane and trimer of hexafluoropropylene [10], methyl ether of 4-oxybenzoic acid and tetrafluoroethylene pentamer [11] . This work is aimed to the synthesis of partially fluorinated alkenaryl ethers based on perfluoro-2-methyl-2-pentene (1) and various derivatives of phenol with the purpose to determine influence of fluorine atoms on biological activity of this class of compounds and intermediate products. It is essential to produce by a way of direct fluorination completely fluorinated alkylcyclohexyl ethers used as high-temperature heat carriers and dielectrics. It is known that when N-nucleophilic reagents react with internal perfluoroolefins in the presence or absence of bases, the isomerization of the starting perfluoroolefins takes place. In the case of perfluoro-2-methyl-2-pentene there is formed perfluoro-2-methyl-1-pentene as an intermediate product, its terminal double bond contains extremely a nucleophilically labile fluorine atom. This condition can considerably influence the direction of the further attack of the nucleophilic reagent and can result in products of a different structure. Thus, another task is arisen: to study the influence of the nucleophilic reagent used and conditions of the process taken into account the properties of the starting internal perfluoroolefin. Earlier it was shown that the interaction of phenol and compound 1 in the presence of KOH in tetrahydrofurane resulted in formation of [3,3,3-trifluoro-1-pentafluoroethyl-2-trifluoromethyl-oxypropenyl[benzene (2), a product of a formal replacement of the fluorine atom at the double bond.
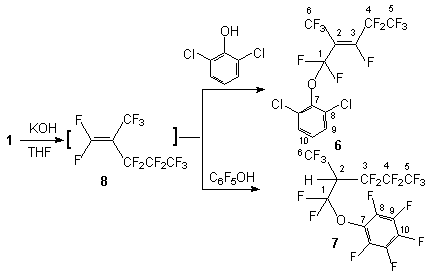
Scheme1 We have determined that the interaction of compound 1 and phenols containing electron donating substituents (2-fluorophenol, 3-metoxyphenol, 3,5-dimetoxyphenol) does not change the character of product formation, there are formed compounds (3-5) respectively. At the same time the reactions of compound 1 with 2,6-dichlorophenol and pentafluorophenol under the same conditions give (1,1,3,4,4,5,5,5-octafluoro-2-trifluoromethyl pent-2-enyloxy)-2,6-dichlorobenzene 6 and (1,1,3,3,4,4,5,5,5-nonafluoro-2-trifluoromethylphenoxy)-pentafluorobenzene 7 respectively. One of explanations of that is the replacement of the fluorine atom in terminal olefin 8 formed from olefin 1 under action of the base.
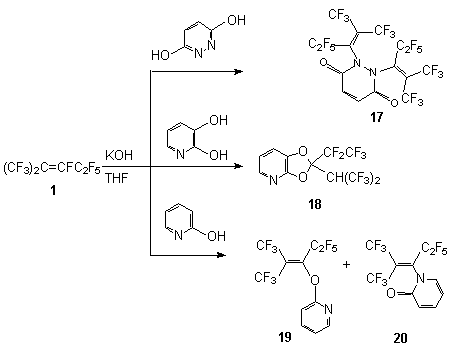
Scheme 2 Such a behavior of these phenols probably is a consequence of the presence of the chlorine and fluorine atoms at the 2- and 6-positions and due to low reactivity of the respective phenolate anions. But the reaction of compound 1 with 2,6-dimethyl-phenol and 2,6-dimethyloxyphenol results in the formation of compounds 9 and 10 despite considerable space requirements. At the same time these results can not be explained by only electron donating properties of the substituents because in the case of interaction of compound 1 with 3-nitrophenol and 4-nitrophenol there are formed mixtures both of the product of fluorine replacement at the internal multiple bond and of the product of fluorine replacement at the terminal bond of intermediate olefin 8 ( compounds 11,12,14,15 respectively). The formation of compounds 14 and 15 is caused by a difference in rates of nucleophilic attack of phenoxy-anion to the carbon atom of the double bond and the rate of isomerization of olefin 1. It is reasonable to expect a similar behavior in the reaction of compound 1 with 3-oxypyridine also, a mixture of compounds 13 and 16 is formed. It was shown that in case of the presence of two OH-groups the reaction involved the two groups with formation of the product of replacement of the fluorine atom at the double bond (compound 17). Thus, interaction of compound 1 and pyridazine-3,6-diol in the presence of KOH in tetrahydrofuran gives 1,5-bis[3,3,3-trifluoro-1-pentafluoroethyl-2-trifluoromethylprepenyl]1,2-dihydropyradizine-3,6-dion 17, whereas the reaction with 2,3-dioxypyridine gives 2-pentafluoroethyl-2(2,2,2-trifluoro-1-trifluoromethyl-ethyl)-[1,3]dioxolo[4,5-b] pyridine 18.
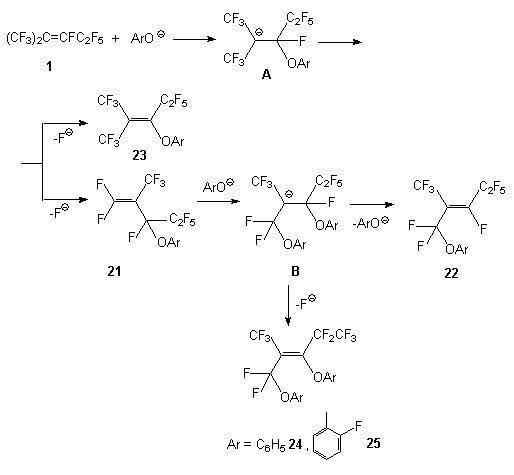
Scheme 3 In the case of reaction of compound 1 with 2-oxypyridine ,as it should be expected, a mixture of 2[3,3,3-trifluoro-1-pentafluoroethyl-2-trifluoromethyl-propenyloxy]pyridine 19 and 1[3,3,3-trifluoro-1-pentafluoroethyl-2-trifluoromethyl-propenyl]1H-pyridine-2-on 20 is formed. That may be explained by the attack of the double bond of olefin1 by O- and N-nucleophilic centers. The reaction of compound 1 with phenol begins by the attack of O-nucleophilic center of the reagent towards the carbon atom of the internal double bond following by generation of intermediate carbanion A stabilized by two CF3 groups. Further transformations of the latter may proceed at the expense of elimination of the fluoride ion both from the CF fragment and from the CF3 group. Comparing thermodynamical stability of the end products, it turned out that the elimination of the fluoride ion from the CF fragment and formation of the product containing phenoxy substituent at the a position to the double bond is more preferable. Prevailing formation of perfluoroolefin 2 in the reaction of compound 1 and sodium phenolate should then be ascribed to kinetic control. However, it must not be ruled out a different way to form compounds 14-16 which may consist in the formation of compound 21 from intermediate carbanion A. In fact, authors [5] have determined the formation of olefin 21 in the reaction between compound 1 and phenol and have isolated such an olefin. The fluorine atoms at the terminal double bond in olefin 21 are more sensitive to the action of nucleophilic reagents comparing with fluorine mobility at the internal double bond of olefin 1. The interaction of olefin 21 with phenolate anion gives intermediate carbanion B. In it stabilization of negative carbanion under action of phenoxy group (OAr) is less effective and there are two ways of stabilization of this carbanion: elimination of the fluoride ion from the CF fragment that brings to reaction products 24 and 25 or elimination of the phenoxy-anion leading to the formation of compound 22. This way is observed exclusively for ArO=OC6F5,OC6H3Cl22 - 2,6; for other phenols containing, for example, NO2 group in the benzene ring a mixture of compounds of 22 and 23 type is formed.
Scheme 4. From our point of view, the way through intermediate formation of carbanion B via olefin 21 seems possible. The structure of new compounds described above has been determined on the basis of the data of IR, 1H, 13 C and 19F NMR spectra (Table 1) and mass spectrometry (Table 2). The IR spectra of these compounds show vibrations of the double bond at 1647 cm-1. The 1H NMR spectrum exhibits signals from hydrogen atom of the aromatic ring in the range characteristic for phenols. In the 19F NMR spectrum the chemical shift and thin structure of the signals correspond to analogs described earlier (see, for example, for phenol[5]), the constants are as follows: JF1F6 is up to 10.7, JF4F6 is up to 23.2, JF5F6 is up to 4.0 Hz.
For compounds of 22 type the 13C and 19F NMR spectra exhibit signals of fluorine and carbon atoms which according to the value of the chemical shift and constants of spin-spin coupling are characteristic for a double bond and fluorine at it. Conclusion. The reaction way for internal perfluoroolefin 1 is determined by the nature of the substituent in the phenyl fragment , that provides a possibility to synthesize partially fluorinated arylalkenyl ethers. Experimental The 19F NMR (ppm) spectra were recorded on a Bruker WP 200SY spectrometer
(188.324 MHz) with C6F6 in CDCl3 as the internal standard. The 13C
NMR (ppm) spectra were recorded on a Bruker-400 spectrometer (100.614 MHz) with
Me4Si ( General synthesis procedure Compound 1 was added dropwise (10.7g, 36 mmol) to a suspension of KOH (2g, 36mmoles) in anhydrous THF (20 mL) at stirring at room temperature and cooled to 0oC. 2-Fluorophenol (4g,36 mmol) was then added in small portions for 15 minutes at stirring, after aging at stirring for 1 hour at 0oC the temperature was raised to room temperature and everything was stirred for another hour and then for 2 hours at 45oC. The reaction mixture was poured into water (100mL), neutralized with diluted hydrochloric acid, extracted with CH2Cl2 (3x30mL) and dried over MgSO4. After distillation of the solvent the residue was distilled in vacuum to produce 2-trifluoromethyl-3-orto-fluorophenoxy-1,1,1,4,4,5,5,5-octafluoropropent-2-en (2). The work has been executed at financial support of STEPI grant (South Korea,2000) and innovational grant N47, 2000 of the Siberian branch of the Russian academy of Science. Table 1. 1H , 13C and 19F NMR Data of the Partly-fluorinated Products
Note : a F in benzene ring; b 98.5 and 97.8 (F1) AB-system JFF = 145.4; c 12.6 (F8), 7.3 (F10), 0.9 (F9) (F benzene ring); d 102.5 (F11,12) 39.1 (F8) 84.2 (F9) Table 2. Yields, Boiling-points and HRMS Data of the Partly-fluorinated Products
Note : a For [M-F]+ References
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Fluorine Notes, 2000, 13, 3-4
 ,0.00) as the internal standard
in CDCl3 (JCH was not measured). The IR spectra were recorded
on a Bruker IFS66 spectrometer (5% in CCl4). The GC-MS spectra were
obtained at 70 eV under electron impact mode and reported as m/z index on a CM
spectrometer Funnigan 8200. The mass spectra were determined on a liquid chromatograph
Hewlett Packard G 1800A, GCD system with a GC electronic ionization detector
and using a 30mm capillary column 0.25 mm covered with a 0.25
,0.00) as the internal standard
in CDCl3 (JCH was not measured). The IR spectra were recorded
on a Bruker IFS66 spectrometer (5% in CCl4). The GC-MS spectra were
obtained at 70 eV under electron impact mode and reported as m/z index on a CM
spectrometer Funnigan 8200. The mass spectra were determined on a liquid chromatograph
Hewlett Packard G 1800A, GCD system with a GC electronic ionization detector
and using a 30mm capillary column 0.25 mm covered with a 0.25  k polymer coating of 5% of diphenyl / 95%
of 1,4-dimethylsiliconate (HP-5), helium as a gas, 1 mL/min, the temperature
of the column was 280oC. The yield, boiling point and HRMS data of
the new compounds are given in Table 1 and Table 2 shows the data of the 1H,
13 C and 19F NMR spectra.
k polymer coating of 5% of diphenyl / 95%
of 1,4-dimethylsiliconate (HP-5), helium as a gas, 1 mL/min, the temperature
of the column was 280oC. The yield, boiling point and HRMS data of
the new compounds are given in Table 1 and Table 2 shows the data of the 1H,
13 C and 19F NMR spectra.