Fluorine Notes, 2000, 10, 5-6
Reactions of fluoroolefins with participation of fluorine inorganic radical initiators.V.M.Andrushin Report 1. Choice of fluorine inorganic radical initiators. Part1. Oxygen fluorides, chlorine oxides, nitrogen fluorides, sulfur fluorides. Development of approaches of physical stimulation of chemical processes has opened new opportunities to synthesize compounds of a desired structure. Development of laser techniques, cryogenic techniques in combination with new chemical technologies allows to generate at low temperatures chemically active reagents (excited molecules, radicals etc.) and is aimed at synthesis of substances inaccessible by conventional chemical syntheses. Reactions of fluoroolefins are not exception, their chemical behavior is specified to a great extent by the presence and character of the reaction initiators. This paper reviews some reactions of synthesis of functional fluoropolymers, perfluorooligoethers and fluoroolefin oxide with radical inorganic initiators in the presence of oxygen. We have examined oxygen-, nitrogen-, sulfur- and halogen-containing covalent fluorides and oxyfluorides as the initiators. The choice of inorganic covalent fluorides as initiators is explained by the existence of the loosely-coupled fluorine atom in their structure and their ability to dissociation, consequently extremely reactive fluorine-containing radicals are formed. The binding energies of fluorine with oxygen, nitrogen, halogens, xenon and krypton are in the range of from 11.7kcal/mol (Kr-F bond in the molecule of the most strong fluorinating agent KrF2) to 70.5 kcal/mol (the N-F bond in the molecules of NF3 and N2F4), while the binding energies of fluorine with electropositive elements are above 100 kcal/mol. Only the binding energies of the S-F bond (78 kcal/mol for the molecule of SF6 and 97 kcal/mol for the molecule of SF4) and the Se-F bond ( 89 kcal/mol in the molecule of Se-F6) are lower than 100kcal/mol.The molecule of BF3has the greatest binding energy, 152 kcal/mol. For comparison, the binding energy of the F-F bond in the fluorine molecule is 36.7 kcal/mol. Oxygen fluorides (OF2,O2F2, O3F2, O4F5) in most cases are difficult to access unstable compounds which were studied at low temperatures. Moreover most of them are toxic and highly explosive. In the synthesis of functional fluoropolymers and fluorinated ethers only oxygen difluoride, OF2 and dioxydifluoride, O2F2 may be of interest as the radical initiators. Oxygen difluoride is a thermally stable ( up to 250oC) toxic gas with a specific objectionable odor. Its reactivity is based on the dissociation: F2O + M Oxygen difluoride is a strong oxidizing fluorinating agent yielding a little in reactivity to elemental fluorine. A comparatively small energy of breaking the F-OF bond ( 42.7 kcal/mol) and easiness of photochemical decomposition at moderate temperatures ( 15-45 oC at 3650A wave length) allow to consider oxygen difluoride as a potential initiator of the reaction of fluoroolefins to produce mono-functional oligomers. Moreover, the patterns of reactions of addition of F and OF fragments to perfluoroolefins to form the appropriate fluoroanhydrides have been described in literature. Dioxydifluoride is an unstable compound, it is decomposed at a temperature above 100oC to fluorine and oxygen according to the scheme: FOOF OOF 2F The energy of breaking the FO-OF bond is 103 kcal/mol and only 18kcal/mol for the F-OOF bond. Thermal decay ( up to 25oC and at a pressure of up to 400Torr) is a homogeneous monomolecular reaction with an activation energy of 17kcal/mol and a half-life of 3 hours at 50oC. Dioxydifluoride is also an effective oxidizing fluorinating agent. In conformity with the aims of our investigation it is essentially that some reactions of dioxydifluoride to form the end OOF-groups have been described in literature. Thus, for example, FSO2OOF was produced in the reaction with SO2, CF3CF2OOF was formed in the reaction with C3F6. CF3OOCF3 was found in the interaction with C2F4. All that allows to expect the synthesis of functional carbon-chain and hetero-chain fluoropolymers on the basis of fluoroolefins. Among chlorine oxides (ClO2, Cl2O3, ClO2, Cl2O4, Cl2O6, Cl2O7) the most perspective for us seem compounds able to generate the OCl radical (ClO2, Cl2O3 and ClO2) which was first found in 1947 and namely its formation explains many reaction mechanisms of oxygen compounds of chlorine. Chlorine monoxide is a gas ( b.p. 2oC, melting point :-121oC) stable up to 100oC. Thermal decay of chlorine monoxide at a temperature of 100-140oC is a homogeneous reaction with formation of the OCl radical. The breaking energy of the Cl-OCl bond is 32 kcal/mol. Chlorine monoxide is an effective chlorination agent. Only its reactions with mono- and dialkylamines among organic compounds have been found in literature, but the fact that hypochlorite is formed in the reaction with water and alkali allows to assume that C-O-C derivatives may be obtained under appropriate conditions. Dichlorotrioxide is stable to 50oC, it is completely decomposed in liquid and gas phases. Its dissociation gives the ClO-radical but its reactions have not been almost studied. It is only known that Cl2O3 catalyzes decomposition of chlorine dioxide, ClO2, while the presence of Cl2O2 slows down the process of dichlorotrioxide, Cl2O3, decay. Chlorine dioxide as well as chlorine monoxide is a gas (b.p.10oC, melting point: -59oC) thermally stable up to 40oC but it is explosive, sensitive to impact, light exposure, heating especially at a temperature above 50oC ( decomposition with an explosion) but there are many approaches to reduce its sensitivity. Chlorine dioxide is an excellent oxidizer (the breaking energy of the OCl-O bond is 55kcal/mol) and most of its reactions run with formation of the ClO-radical. Nitrogen fluorides (NF3, isomers N2F2,N2F4,FN3,HNF2,CINF2, Cl2NF) at standard conditions are toxic gases. Some of them are explosive particularly at phase changes. Among them only nitrogen trifluoride, tetrafluorohydrazine and chlorodifluoroamine are of interest as inorganic initiators of reactions of conversions of fluoroolefins. They are able to homolytic breaking the bond R-NF2 with formation of difluoroamino-radicals and F, NF2, Cl radicals respectively. The NF2 radical is stable enough: an average binding energy is 67 kcal/mol, it is in equilibrium with its own dimmer, tetrafluorohydrazine. Nitrogen trifluoride is least reactive and most thermally stable among all nitrogen fluorides: up to a temperature of 300-350oC it reacts very slowly and only with aqueous solutions. He is a weak fluorinating agent ( the energy of breaking the F-NF2 bond is 55 kcal/mol), and a difluoroamination agent with fluorine acceptors. The reactions of addition of nitrogen trifluoride to fluoroolefins are known, but high reaction temperatures do not allow to expect its application as the initiator. Tetrafluorohydrazine is a toxic gas (b.p. 74oC, melting temperature: -161oC), stable up to 300oC, first synthesized in 1958. Chemical behavior of tetrafluorohydrazine is determined by its equilibrium homolytic dissociation to the radicals: N2F4 More than ten papers has been devoted to the study of that equilibrium and determination of its thermodynamic characteristics. The energy of breaking the N-N bond is 20.5 kcal/mol and 70.5 kcal/mol for the F-N bond, that determines easiness of formation of difluoroamino-radicals on the one hand and their high stability on the other hand. An increase in temperature and a decrease in pressure shift the equilibrium to the right hand side. Thus at a temperature of 55oC and a pressure of 260 Torr the dissociation degree is 0.94% and the content of the difluoroamino radical is 1.8% , but at a temperature of 150oC and a pressure of 5 Torr the dissociation degree is 93% and the radical content is 96.4%. In most reactions the rate of the tetrafluorohydrazine homolysis exceeds significantly the main reaction rate , therefore the concentration of the NF2 radicals does not depend on the passing the main reaction being the interaction of the NF2 radical with other reagent. Tetrafluorohydrazine is added to olefins to form bis(difluoroamino)alkanes. In a number of reactions the difluoroamino radical behaves as a pseudo halogen. Chlorodifluoroamine under standard conditions is a gas ( b.p.: 67oC, melting temperature: -190oC). Its reactivity is determined by the homolytic dissociation according to the scheme: ClNF2 At a temperature above 100oC, irreversible decomposition takes place according to the reaction: 6ClNF2 4NF3 + N2 + 3Cl2 ClNF2 decomposition runs already at room temperature and strongly depends on the material of the reactor walls. Chlorodifluoroamine is a strong chlorinating and difluoroamylating agent. The energy of breaking the Cl-NF2 bond is 35kcal/mol. As a result of the reaction of chlorodifluoroamine with olefins, vinyl bis(difluoro)amino- and dichloro-derivatives are formed as well as adducts, that has been confirmed by the radical character of the reaction similarly to the reactions of tetrafluorohydrazine. Sulfur fluorides (SF4,SF6,S2F2, S2F10) are not of practical interest as the initiators for the reaction of fluoroolefins and so are not considering here. The analysis of possible initiators from the group of fluorides of oxygen, nitrogen, sulfur, chlorine oxides has shown that the most acceptable compounds for practical application are the following: oxygen difluoride, F2O; dioxydifluoride, F2O2; chlorine monoxide,Cl2O; dichlorotrioxide, Cl2O3; chlorine dioxide, ClO2; nitrogen trifluoride, NF3; tetrafluorohydrazine, N2F4 and chlorodifluoroamine, ClNF2. Part 2 of the present report will review possible initiators from a group of oxyfluorides of nitrogen, halogens, sulfur and a choice will have been done for the most perspective initiator for the fluoroolefins reaction |
Fluorine Notes, 2000, 10, 5-6
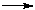 F + OF + M.
F + OF + M.