Fluorine Notes, 2000, 8, 11-12
Development of modified compression oils with fluorine-containing fragments in the main chain
L.M. Popova, O.A.Prytkova, V.V.Drozdov,N.Ya.Rabinin
The signing of the Montreal Protocol by Russia has become a powerful incentive to search for routes of synthesis of oligo-ethers possessing solubilizing ability towards ozone safety chladones with the purpose of their application as compression oils [1].
A method of synthesis based on the reaction of nucleophilic addition of  -olefins (hexafluoropropene) to polyoxaalkylen glycols (laprols 402 and 502) has been proposed with the purpose to develop new high-performance compression oils with improved operating characteristics, particularly with lower coefficients of kinematic viscosity,
-olefins (hexafluoropropene) to polyoxaalkylen glycols (laprols 402 and 502) has been proposed with the purpose to develop new high-performance compression oils with improved operating characteristics, particularly with lower coefficients of kinematic viscosity, 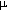 , and dissolving alternative chladones over a wide range of operating temperatures of –40/20oC [2].
, and dissolving alternative chladones over a wide range of operating temperatures of –40/20oC [2].
Interaction of n-propyl alcohol with hexafluoropropene was taken as an example to study the nucleophilic addition of -olefins to polyoxaalkylen glycols. The reaction was carried out in a glass reactor, in which n-PrOH and solid caustic potash ( 0.1 equiv) was charged and hexafluoropropene was bubbled at 40oC for 4-6 h. A mixture of products (1,2) was produced under these conditions in a 98% yield. Disappearance of the absorption band
-olefins to polyoxaalkylen glycols. The reaction was carried out in a glass reactor, in which n-PrOH and solid caustic potash ( 0.1 equiv) was charged and hexafluoropropene was bubbled at 40oC for 4-6 h. A mixture of products (1,2) was produced under these conditions in a 98% yield. Disappearance of the absorption band  OH at 3350cm-1 in the IR spectra and appearance of the characteristic bands in the range of 900-1200 cm-1 was the evidence of the reaction completion, more over bands of valence vibrations of multiple bond C=C at 1758cm-1 were observed that was also confirmed by the data of 19F NMR spectroscopy. That is an evidence of passing a competing reaction of dehydrofluorination of compound (1) in the presence of alkali which results in the formation of product (2) [3,4]:
OH at 3350cm-1 in the IR spectra and appearance of the characteristic bands in the range of 900-1200 cm-1 was the evidence of the reaction completion, more over bands of valence vibrations of multiple bond C=C at 1758cm-1 were observed that was also confirmed by the data of 19F NMR spectroscopy. That is an evidence of passing a competing reaction of dehydrofluorination of compound (1) in the presence of alkali which results in the formation of product (2) [3,4]:
CH3CH2CH2OH + CF2=CFCF3  CH3CH2CH2OCF2CHFCF3(1)+ CH3CH2CH2OCF=CFCF3(2)
CH3CH2CH2OCF2CHFCF3(1)+ CH3CH2CH2OCF=CFCF3(2)
By analogy with producing 2-N-(perfluoropropyl)propyl ether (1) and its unsaturated analogue (2), products (3,4) were produced by interaction of polyethylene glycol (PEG) with hexafluoropropene under the same experimental conditions (10% KOH, 40oC, 4-5h). The yield attained 18%. Products (3,4) were isolated and dried in vacuum, a light yellow oily liquid was formed as the result. The data of IR and 19F NMR spectroscopy witness that the reaction of dehydrofluorination takes place. Appearance of the absorption band  C=C at 1770cm-1 was observed in the IR spectrum.
C=C at 1770cm-1 was observed in the IR spectrum.
HO(C2H4)nH + CF2=CFCF3  CF3CHFCF2O(C2H4O)nOCF2CHFCF3(3) +
CF3CHFCF2O(C2H4O)nOCF2CHFCF3(3) +
CF3CHFCF2O(C2H4O)nOCF=CFCF3(4) + CF3CF=CFO(C2H4O)nOCF=CFCF3 (5)
A temperature increase up to 175oC during several days resulted in an increase of the intensity of the absorption band in the IR spectra assigned to valence vibrations of the C=C bond at 1770cm-1 and appearance of a new absorption band at 1655cm-1 probably assigned to the formation of compound (5).
Coefficients of kinematic viscosity for aggregate products (1,2) and (3,4) have been determined: 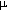 22(PrOH)2.23cst and
22(PrOH)2.23cst and 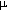 22(1,2) 1.98cst;
22(1,2) 1.98cst; 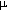 22(PEG)175.95cst and
22(PEG)175.95cst and 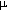 22(3,4)141.68cst.
22(3,4)141.68cst.
Thus, a principal possibility to modify hydrocarbon oils with the purpose to increase their solubilizing ability towards alternative chladones by means of introduction of fluorine-containing fragments to the main chain of hydroxyl-containing and polyoxaalkylen glycols has been shown on the example of the reaction of nucleophilic addition of hexafluoropropene to the model n-propyl alcohol and polyethylene glycol. A limitation of this method is the formation of noticeable amounts of unsaturated compounds (2 and 4) and a big consumption of hexafluoropropene. In this connection, a saturated solution of KOH was used as a catalyst and the process was carried out in a metal ampoule under overpressure at 40-50oC.
This procedure was successfully used to obtain modified laprols 402 ( polyether based on ethylene oxide) and 502 (polyether based on propene oxide and ethylene oxide). The process was carried out in a steel cylinder of 1 L capacity . 200 mL(0.5 mol) of laprol 402(502) and 12 mL of 12% solution of KOH were charged into the cylinder, an excess of hexafluoropropene was then added at 40-50oC at vigorous stirring up to complete absorption of the monomer. Varying the amount of hexafluoropropene, a modified product with a desired number of perfluoro-containing units in the molecule of polyoxapolyalkylen glycol was obtained. The conversion degree attained 99%. Then the reaction mass was discharged, washed with ice water to pH 7 and dried under vacuum over P2O5. The coefficients of kinematic viscosity of the initial laprol 402 and modified oil were respectively 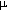 22(L402) 90cst and
22(L402) 90cst and 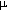 22(modif.L402) 67.9cst. An ion-exchange column was used to remove excess fluoride-ion which content attained 0.2% in the modified product.
22(modif.L402) 67.9cst. An ion-exchange column was used to remove excess fluoride-ion which content attained 0.2% in the modified product.
The IR spectra showed absorption bands  C-H at 2800cm-1,
C-H at 2800cm-1,  C=C at 1750 cm-1 and characteristic absorption bands
C=C at 1750 cm-1 and characteristic absorption bands  C-F in the region of 900-1200cm-1 [3].
C-F in the region of 900-1200cm-1 [3].
The analysis of the PMR spectra of the initial laprols 402 and 502 has confirmed the presence of oxaethylene and propene fragments. Chemicals shifts of methylene ( 3.4-3.5 ppm) and methyl (
3.4-3.5 ppm) and methyl (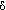 1.06 ppm) groups were determined but the resonance peaks are broaden as a result of overlap between neighbor signals of magnetically equivalent CH3 and CH2groups. That did not allow to determine values of spin-spin interaction coefficients.
1.06 ppm) groups were determined but the resonance peaks are broaden as a result of overlap between neighbor signals of magnetically equivalent CH3 and CH2groups. That did not allow to determine values of spin-spin interaction coefficients.
In the 19F NMR spectra of modified laprols 402 and 502 the following signals were observed: doublet-doublets with chemical shiftd 75.7 ppm for CF3group, doublet-quartets at 42ppm from CHF group and quartet of AB-system at 79 ppm ( with respect to CFCl3).
The presence of CHF group in the PMR spectra of compound (3) has been confirmed by the proton resonance signal at 4.15 ppm (JFH 56Hz: JCF3CH 6Hz)(with respect to TMS). More over, analysis of 19F NMR spectra discovered signals corresponding to dehydrofluorination product (4), CF-CF3 groups with chemical shift at 193 ppm and CF-O fragment at 108.5 ppm (with respect to CFCl3).
For express-estimation of the degree of addition of hexafluoropropene to laprol 402, a method of subsonic structurometrybased on determination of structure parameters almost without imperfection (elastic deformation or plastic yielding, or their combination). The method was tested for laprol 502 and modified product and for laprol 402 also. The value of plastic viscosity coefficient for modified laprol 502 is 6 times lower than that for the initial one. Such a significant difference in values allows to use this method for express-analysis of the degree of addition of hexafluoropropene to laprols.
Antifriction properties of oils produced as a result of hexafluoropropene addition to laprols, data of measurements conducted at the RSC “Applied Chemistry” are given in the Table
Table. Antifriction properties of modified laprol 402
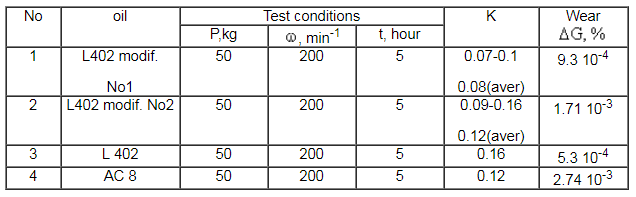
Tribilogical behavior of modified oils containing fluorinated terminal links in the main chain have been investigated at the Institute of reliability of components (Minsk city). It has been shown that the friction coefficient is decreased 2.5 times and wear resistance is increased by 15-25%.
As a result of the conducted investigations, a general method to modify polyoxapolyalkylen glycols with hexafluoropropene allowing to improve performance characteristics of hydrocarbon oils has been developed.
References
- Abstracts of the 1-st and 2-nd International Conference “ Chemistry, technology and application of fluorocompounds”, May,30-June,3,1994; Sept. 23-26, 1997 St.-Petersburg. 207pp., 169pp..
- A.Lovelase.D.Rouch, U. Postelnek. Aliphatic fluorine-containing compounds/M//IL/1963/p.156-158
- L.A.Kazitsina, N.B.Kupletskaya. Application of UV-, IR- and NMR spectroscopy inorganic chemistry/M/VSh/1971/p.264
- L.M.Popova, V.V.Vold’kin,O.A.Prytkova, V.V.Drozdov, O.M.Tsvetkov, N.A.Riabinin. Abstr. 2-nd Intern.Conf. of Chemistry, Technology and Applications of Fluorocompounds/ Sept.23-26,1997/St.-Petersburg/Russia/1997/ p.123
Fluorine Notes, 2000, 8, 11-12
