Fluorine Notes, 1999, 7, 5-6
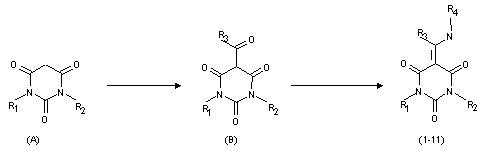
Synthesis of 5-aminomethylenbarbituric acids with fluoroaromatic substituents.V.T.Papp, K.A.Krasnov, S.V.Vershilov Derivatives of barbituric acids are known as substances showing cytostatic, sedative, antivirus, herbicide and other types of biological activities [1-6]. A perspective direction to modify properties of various substances ( including medicines) is to introduce a fluorine atom or a fluorine-containing fragment into their structure[7-9]. In this connection, synthesis of derivatives of 5-aminomethylenbarbituric acids containing fluoroaromatic substituents is of undoubted interest. By the moment of the research beginning , we could not find any data on their producing. The aim of this study was to develop methods of synthesis of fluoroaryl derivatives of 5-aminomethylenbarbituric acids ( Fig.) In order to produce derivatives (1-11), usually, either method of condensation of substituted ureas with appropriate derivatives of malonic acid in the presence of sodium ethylate or methods based on interaction of barbituric acid or N-substituted barbituric acid with formylating agent and the appropriate alkylamine as well as condensation of barbituric acids with formamides and various modifications of the above methods [6]. Formyl-derivatives of barbituric acids were produced by treatment of the appropriate barbituric acids with dimethylformamide at boiling point or according to the Vilsmeir reaction under most mild conditions.
Fig. R1=H(1),CH3 (2,3,4,5,10,11), C6H5 (6,7), C6H5CH2 (8,9) R2=H(1,2,3,6,7,8,9),CH3(4,5,10,11) R3=H(1,2,4,6,8,10), CH3 (3,5,7,9,11) R4=p-FC6H4 (1-9), C6F5NH(10,11) Acylation of barbituric acids with acetic anhydride was carried out in the presence of sodium bicarbonate (pH 7.5-8.0). In this way of acylation, the reaction product precipitates and is readily separated from the reaction mixture by filtration. The yield is 60-80% of the theoretical value. Condensation of 5-acetylbarbituric acids with amines was carried out under various conditions chosen for each individual experiment. Alcohols, alcohol-water mixtures, water were used as a solvent. Most products were well produced in water alcohol at short heating (50oC). The yields attained 60% of the theoretical ones and depended mainly on solubility of the goal product in the reaction mixture. Purity of the products obtained was tested by thin-layer chromatography method in various systems of elutriators ( depending on the substance nature). The structure and composition of the synthesized substances were determined by means of elemental analysis and data of PMR spectroscopy ( Table) Table.Properties of 5-aminomethylenbarbituric acids
Experimental PMR spectra were recorded on a AM-500 Bruker NMR-spectrometer. The starting substituted ureas were synthesized according to known methodics [10-12] or used as commercially provided (Merck,Lancaster) Substituted barbituric acids were produced according to the known method from the appropriate substituted ureas by condensation with malonic ester in alcohol solution using sodium alcoholate or lithium hydride as a condensing agent [13]. Barbituric acids containing steric hindrance substituents and di-substituted barbituric acids were produced from appropriate ureas and malonic acid using acetic anhydride, phosphorus oxychloride or phosphorus pentachloride as a condensing agent [14,15]. Formylbarbituric acids were produced by heating barbituric acids with a small excess of dry dimethylformamide at boiling point or according to the Vilsmeier reaction at the most mild conditions. Acetylbarbituric acids. 3 g(0.015M) of 1-phenylbarbituric acid was dissolved in 15 mL of 10% water solution of Na2CO3. 1.5M of acetic anhydride was added at once at vigorous stirring and almost immediately elimination of carbon dioxide started. The reaction mixture was allowed for stirring till the formation of a flaky residue then stirring was continued for 30 minutes more. The precipitate was filtered and washed on a filter to neutral reaction. The product was dried in air at room temperature. 5-Aminomethylen (or ethylidene-)-barbituric acids were synthesized by condensation of 5-formyl-(or acetyl-) barbituric acids with primary amines: 0.005 M of formyl-(or acetyl-)-barbituric acid was dispersed in 15-25 ml of water alcohol and primary amine, pure or dissolved in a small amount of water alcohol, was added at stirring. Dissolving the starting substances was observed and the reaction product then started to precipitate. The reaction mass was kept at the reaction temperature for 15-30 minutes then cooled and the reaction product was filtrated and washed on a filter with water alcohol. The washed precipitate was dried in air at room temperature. As a rule the product was clean enough but it may be reprecipitated from the corresponding solvent if necessary. The yield was 30-60% of the theoretical value. References
|
Fluorine Notes, 1999, 7, 5-6