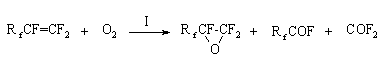Fluorine Notes, 1999, 6, 3-4
Initiated oxidation of perfluoroolefinsV.A.Soshin, L.G.Tihonova, G.I.Lekontseva Oxidation of perfluoroolefins (PFO) is of practical interest as a method to produce perfluoroepoxides (PFE) and perfluoropolyethers (PFPE) used in production of polymeric fluorine- and oxygen-containing materials possessing unique properties which meet the requirements of a number of modern branches of technology [1,2]. PFO oxidation with chemical reagents, hydrogen peroxide [3], hypohalogenites of alkali metals [4], potassium permanganate [5] is well known. Methods of PFO oxidation with molecular oxygen, as a rule, under the influence of activation of various types such as photochemical irradiation, catalysts, high temperature under pressure [6-9] are mostly used. The role of activators is to create conditions for primary formation of free radicals initiating radical chain process of PFO oxidation. All the mentioned methods have specific disadvantages hampering their access to development of industrial technologies. In this connection it was of interest to expand methods of PFO initiation with molecular oxygen. Halogens (fluorine, chlorine, bromine) and trifluoromethylhypofluorite (TFMH) were chosen as the object for the study as substances able to generate active sites at moderate temperature in the absence of UV-irradiation or radiation. This choice is based on the results of investigations of reactions in a system of C2F4 + Cl2 +O2 which demonstrate the role of chlorine in formation of peroxide radical [10,11] and comparable dissociation energies of halogens [12] and TFMH [13]. Experimental The PFO oxidation was carried out in flow reactors of metal and nonmetal materials in gas phase or in a medium of chlorofluorocarbon liquids (R-318, 12F, 11F,13F liquids) at atmospheric pressure. A reactor for gas-phase oxidation was made as a spiral of Teflon or glass pipe of 800 mm length, 8mm diameter placed in a liquid thermostated bath. Raw materials are fed through a tee. A reactor for liquid-phase oxidation is a glass vertical cylinder of 600mm height, 50mm diameter equipped with a jacket for heat carrier circulation. Raw materials are fed under the liquid layer through pipes placed axially. The first representatives of PFO homologous series, tetrafluoroethylene (TFE) and hexafluoropropylene (HFP), were chosen for the study. A mixture of equal volumes of oxygen and nitrogen containing initiator was used as TFE oxidizer. HFP was oxidized with a mixture of oxygen and initiator. The initiator concentration in the oxidizing mixture was studied in a range of 0.5-10.0 vol.%, most experiments were carried out at a concentration of the initiator of 1.5-2.5 vol.%. The content of the oxidizing and reaction gas mixtures was determined by GL chromatography method using a column of 3m length, 4mm diameter at a temperature of 20oC and a rate of helium gas-carrier of 1.3L/h and 2.0 L/h respectively. For the oxidizing mixtures the column was filled with silochrom stabilized with PFO oxidation products, and with polysorb containing 5% of FS oil for the reaction gas mixture. This method allows to determine the content of PFO, perfluoroepoxides and by-products such as perfluorocyclopropane (PFCP), hexafluoroacetone (HFA) and total fluoroanhydrides (FA), carbonylfluoride (CF) and trifluoroacetylfluoride (TFAF). Separate determination of CF and TFAF was carried out according to the data of IR spectroscopy in a range of wavelengths of 1930-1940 cm-1 and 1900 cm-1 respectively. In the balance experiments with the purpose to determine the yield of PFE, the reaction gases were condensed in a trap cooled with a mixture of ethyl alcohol and liquid nitrogen to temperature of –90oC and subjected to low-temperature rectification to separate individual products. Discussion of experimental results. The experiments on TFE and HFP oxidation initiated with halogens and TFMH were carried out in reactors of heat resistant glass at a mole ratio of PFO/oxidizing mixture of 1/1 (Tables 1,2) Table1. Initiated oxidation of TFE at a temperature of 20-25 o C and initiator concentration of 1.5-2.0% by volume
Analysis of the experimental data has shown that PFO oxidation in the presence of the proposed initiators allows to synthesize the appropriate epoxides together with products of more complete oxidation. The data of Tables 1,2 have shown the advantage of liquid phase oxidation method with use of TFMH as the initiator, leading to an increase in the epoxide yield and milder temperature conditions of the reaction. During the investigation of the reactor material influence on the yield of the reaction products, experiments on gas phase and liquid phase oxidation of HFP were carried out at a temperature of 80-100oC, TFMH concentration in oxygen of 1.8% by volume, the HFP:O2 molar ratio of 1:1, contact time of 7.5-8 minutes (Table3). Table 3. Results of the HFP oxidation in dependence on the reactor material
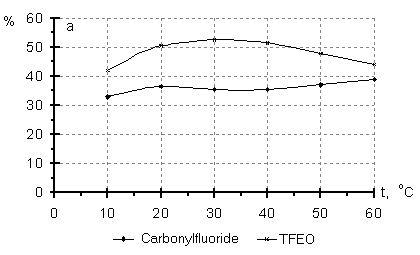
It is seen from Table3 that direction of the initiated oxidation in gas phase depends considerably on the reactor material. A higher epoxide yield was observed in the nonmetal reactors, while more complete HFP oxidation or HFPO isomerization to hexafluoroacetone took place in the metal reactors. The catalytic effect of the reactor wall became apparent to a less degree when the reaction was carried out in liquid phase. These results are in accord with reference data [14] on HFP liquid phase oxidation under pressure in reactors of stainless steel and Teflon. Taken into account the above mentioned, it is of interest to study regular dependencies of PFO interaction with molecular oxygen. Experiments were carried out in the nonmetal reactors with using TFMH as the reaction initiator. According to reference data [6], the direction of the PFO oxidation reaction depends to a great degree on the activation energy determining temperature conditions of the process. The results of the initiated liquid phase oxidation of TFE and HFP at different temperatures are given in Fig.1. It is obvious from the mentioned data, that an increase in the content of epoxides in the oxidation products is observed in a certain temperature range, a temperature above this range brings to a decrease in the concentration of epoxides because of an increase in amount of by-products of more complete PFO oxidation. Fig.1 Content of the products of liquid phase oxidation of TFE (a) and HFP (b) versus temperature
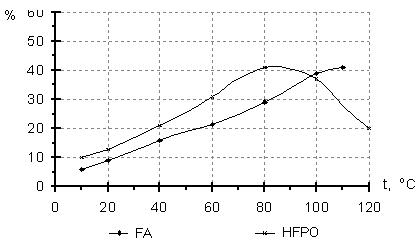
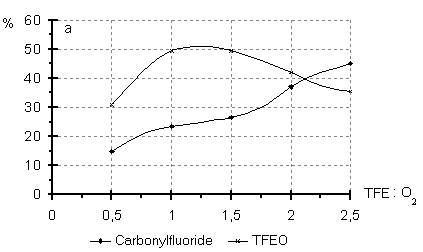
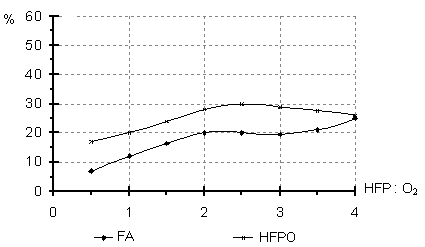
A number of experiments was devoted to investigation of the PFO:O2 molar ratio influence on the composition of the oxidation reaction products (Fig.2).In the TFE oxidation an increase of the TFE:O2 ratio over 1:1-1.5 brings to a decrease in the TFEO content at the expense of CF concentration growth. In the HFP oxidation the increase in the HFP:O2 molar ratio to 1:2-4 does not affect much the composition of the oxidation products. Fig.2 Content of the products of liquid phase oxidation of TFE (a) and HFP (b) versus molar ratio
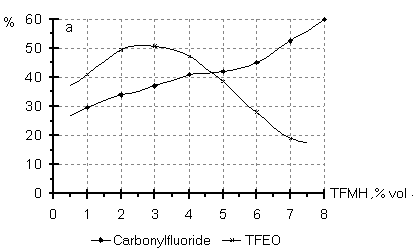
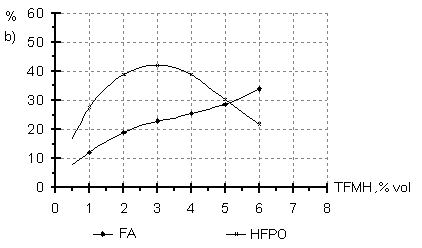
Dependence of the reaction direction on the initiator (TFMH) concentration in the oxidizing mixture was studied in the TFE oxidation in R-318 at a temperature of 40oC and the molar ratio of TFE:O2:N2= 1:1:1 (Fig.3). A growth of TFMH concentration in the oxidizing mixture to a value of 1-4 vol.% does not affect significantly the reaction direction, further a decrease in the TFEO content is observed, probably, due to a blocking of reactive sites accounting for epoxide formation. Fig.3 Content of the reaction products of the liquid phase oxidation of TFE (a) and HFP(b) versus the initiator concentration
A similar picture is observed also in HFP oxidation in a medium of 12F chlorofluorocarbon liquid at 900C and the HFP:O2 molar ratio equal to 1:1 (Fig.3). As a result of the conducted experiments, it has been shown that the synthesis of TFEO and HFPO is preferably to carry out in liquid phase in nonmetal reactors at optimal temperatures of 20-40oC for TFE and 80-100oC for HFP , at the molar ratio of PFO:oxidizing mixture in the range of 1:1-1:2 and the initiator concentration of 1.0-4.0 vol.% ( TFMH preferably). The content of PFO oxidation products and regular dependencies of their formation
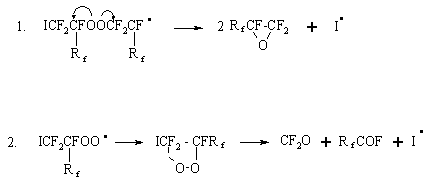
allow to assume that the reaction takes place according to radical-chain
reaction mechanism (this is in accord with literature data [4,12,13]) and
can be presented by common scheme of the initiated PFO oxidation where free
radicals I2
CF3OF
Further under action of the dissociated initiator and PFO, a free radical is formed, it gives the start to the chain process of oxidation with intermediate formation of peroxides and polyperoxides:
There are two routes of the intermediate products stabilization depending on the oxidation conditions [13]:
According to the proposed scheme in the optimal oxidation regime , there are conditions for formation of polyperoxides and further perfluoroepoxides as the main reaction products:
where Rf=F or CF3. Under more harsh conditions (elevated pressure, oxidizer excess, catalytic effect of the reactor walls) stability of the primary peroxide radical decreases and mainly side reactions take place. In principle the important characteristic of the initiated reactions is a possibility to carry out the oxidation in flow systems without pressure at sufficiently moderate temperatures, that makes them very perspective in development of technological processes. The data obtained have been assumed as a basis of the development of pilot technology to produce HFPO. Conclusions.
References
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Fluorine Notes, 1999, 6, 3-4
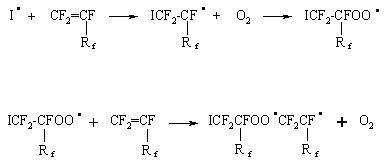 and more
and more