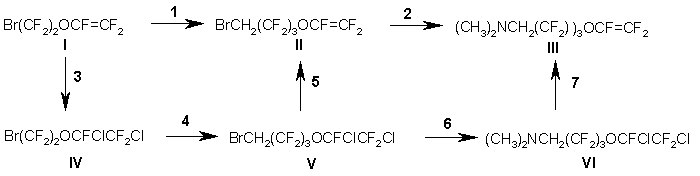Fluorine Notes, 1999, 5, 9-10
Synthesis
and application of |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element | Starting polymer,atom% | Aminated sample, atom % | |||||
| film | granules | ||||||
| Calc. | Found | Calc. | 50oC, 5h |
100oC,
5h |
130oC,1h | 50oC,5h | |
|
C |
34.2 |
30.00 |
36.6 |
24.42 |
- |
- |
25.51 |
|
O |
2.7 |
10.39 |
2.4 |
7.87 |
- |
- |
3.62 |
|
Br |
2.7 |
1.11 |
2.6 |
2.28 |
2.98 |
2.19 |
1.01 |
|
F |
60.4 |
58.5 |
55.9 |
61.87 |
95.88 |
95.39 |
67.54 |
|
N |
- |
- |
2.5 |
3.58 |
1.14 |
2.02 |
2.27 |
Note: hydrogen atom is not defined by X-ray photoelectron spectroscopy method
According to the results of X-ray photoelectron analysis one can conclude that substitution of Br atoms with N(CH3)2 group was performed most completely at 40-50oC. Here the results of the analysis of film and granules `witness that at 50o substitution of bromine atoms with the dimethylamine groups in granules was performed more completely in comparison with film ( Br content was 1.01 and 2.23% respectively). But according to the ratios of the atom content of C,O,F,N and their calculated values one can assume that partial destruction of the polymer took place in granules as well.
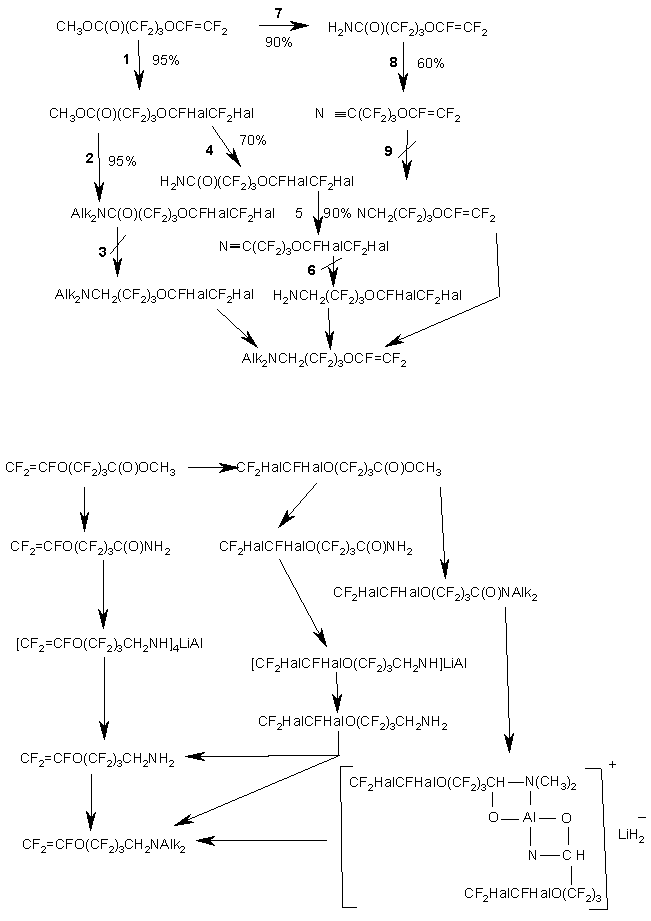
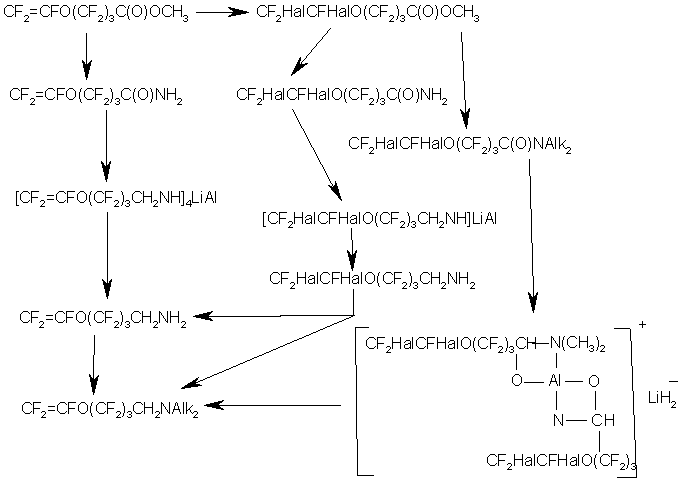
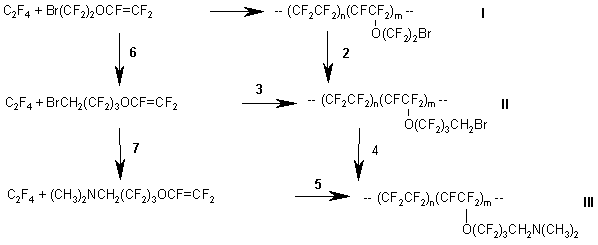
Similarly to the synthesis of VEA monomers, the formation of homopolymer in large amounts also took place in this scheme in stage 2 when vinylidene fluoride was used. It should be noted regarding other stages that the yields were high enough for technological development of the process. The synthesized monomers were used to make membranes, which displayed high efficiency in electrolytic processes.
Thus, a fundamental possibility to create fluoropolymers with specified properties
by the alternative route using active
![]() -
bromoalkylvinyl ethers has been shown. The produced samples of materials
were found to possess anion conductivity and chemical resistance in strong
acid environment in the presence of active oxidizer at elevated temperatures.
-
bromoalkylvinyl ethers has been shown. The produced samples of materials
were found to possess anion conductivity and chemical resistance in strong
acid environment in the presence of active oxidizer at elevated temperatures.
References
- L.V.Shevchenko,N.A.Riabinin,V.M.Andrushin. The fluorinated alkylvinyl ethers and polymer materials on their base. Review information. S.-P.,1992
- I.S.Kozhuhova, N.A.Riabinin, S.V.Koreneva. The perfluorovinyl ethers for fluoropolymers, elastomers and rubbers. Patent study. S.-P.,1992
- R.E.Kesting. The synthetic polymer materials. M., Himiya,1991.336p.
- N.Isikava. Novelty in the technoligy of fluorine compounds. M.:Mir,1984,p.337-340
- Synthesis of organofluoric compounds. Monomers and intermediate products/ Collected articles,M.:Himiya,1977
- V.M.Andrushin,N.B.Pavlova. Fluorine notes.1999,N4.
Fluorine Notes, 1999, 5, 9-10