Fluorine Notes, 1999, 5, 3-4
Conversions of perfluoro-substituted 2-amino-4-methylpyrimidines in the oxidation reaction with potassium permanganate in alkali environment.L.M Popova, A.Yu.Trishina,S.V. Vershilov Oxidation of 6-perfluorosubstituted 2-amino-4-methylpyrimidines with potassium permanganate in alkali environment has resulted in the formation of the appropriate 2,2'azobis-derivatives of pyrimidines. High cost of fluorine introduction into a molecule is a problem in application of fluorine-containing diazo compounds as potential synthons in production of dyes and biologically active substances. But the unique properties introduced by fluorine, which impossible to attain with the assistance of different elements, contribute to development of organofluorine chemistry, and respective cost reduction of fluorine-containing compounds will allow to expand application area of this class of compounds.
To continue investigations in the area of synthesis and study of properties
of derivatives of fluorine-substituted azines and azoles [1,2], we have
carried out the reaction of oxidation of 2-amino-4-methyl-6-perfluoro-substituted
pyrimidines (I-V) with potassium permanganate in alkali environment,
as a result azobis-derivatives of diazines (VI-X)have been separated
and described. Earlier the mentioned 2-amino-pyrimidines (I-V) were reported
to be produced by cyclization of asymmetric fluorine-containing
The analysis of literature data [3,4] has shown that introduction of an electron-deficient substituent, perfluorinated fragment in particular, into a dye molecule increases light-resistance and brightness of dyeing.
Perfluoroalkyl group as a substituent in a dye molecule displays strong -I-effect
spreading through
Azocoupling reactions of various substituted pyrimidines with diazonium salts are widely presented in literature [5]. They are remarkable for variety of reaction conditions affecting the position of azo-group introduction in the pyrimidine ring. It is necessary to remember that diazonium salts react with primary amino groups with formation of diazoamino-products.
One of the ways to synthesize azopyrimidines is diazotization of 2,4,5,6-tetraaminopyrimidines
according to standard procedure to 2,4,6-triaminopyrimidyl-5-diazonium
chloride following by azocoupling of the intermediate produced with 2,4,6-triaminopyrimidine
in acetate buffer at pH 5.5. As a result of the reaction, 2,4,6-triamino-5-(2',4',6'-triamino-pyrimidyl)azopyrimidine
was yielded in 63%[6]. Attempts to find convenient procedures of separation
and purification failed, thus the formation of azopyrimidine was registered
by UV-spectroscopy method. Absorption maxima in the area of 256,276,390
nm are characteristic for the electron spectra. Appearance of the absorption
bands in the short-wave range of the azopyrimidine spectrum (probably,
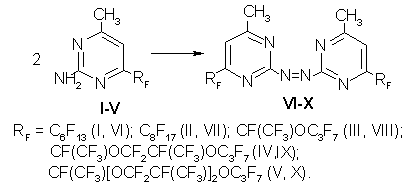
I n the course of the study we synthesized 2,2'-azobis(4-methyl-6-perfluorosubstituted pyrimidines((VI-X) by oxidation of the appropriate 2-amino-derivatives of diazine (I-V) with aqueous-alcoholic solution of potassium permanganate in alkali environment at room temperature for 6 h. Products of azocoupling (V-X) were separated after the reaction mass treatment with 5% solution of hydrochloric acid to pH 4-5 and following extraction with R-113. After distillation of the solvent and drying, the yield of compounds (V-X) attained 60-70%. Perfluoro-substituted azobispyrimidines were separated as viscous oils of a characteristic smell. IR-spectra of 6-perfluorosubstituted azobis-derivatives of pyrimidine (VI-X) presented absorption bands of valence vibrations of methyl groups in a range of 3392-2850cm-1, skeleton vibrations of heteroring are characterized by absorption bands in a range of 1770-1405 cm-1 and valence vibrations of C-F bond become apparent at 1350-890cm-1.
Experimental The IR-spectra were recorded on a Shimadzu IR-470 (Japan) instrument (thin film). Control over the course of the reactions was conducted by thin-layer chromatography method on a Sulifol UV-254 plates. Starting 6-perfluorosubstituted 2-amino-4-methylpyrimidines (I-V) were synthesized according to method[1]. 2,2'-Azobis (4-methyl-6-perfluorohexylpyrimidine)(VI). To a solution of 1.2 g (0.0028 mole) of 2-amino-4-methyl-6-perfluorohexylpyrimidine (I) in 10 mL of ethanol a solution of 0.9g (0.014 mole) of potassium hydroxide in 20 mL of water was added dropwise. A solution of 0.44g (0.0028 mole) of potassium permanganate in 15 mL of water was then added and kept for 4h. The reaction mass was treated with 5%solution of hydrochloric acid to pH 4-5, extracted with R-113, washed with water, dried, the solvent was distilled. The yield was 0.7g (60%), a yellow oily substance. IR spectrum, thin layer (cm-1): 3280,3180,1740,1680,1610,1580,1350,1145. 2,2'-Azobis(4-methyl-6-perfluorooctylpyrimidine)(VII). It was produced similarly to compound (VI) from 2.1g(0.004 mole) of 2-amino-4-methyl-6-perfluorohexylpyrimidine (II). The yield was 1.3g (63%),yellow oily substance. IR-spectrum, thin layer (cm-1):3392,2915,2870,1770,1685,1550,1460,1390-890. 2,2'-Azobis [4-methyl-6-perfluoro(1-methyl-2-oxapentyl)pyrimidine] (VIII).It was synthesized analogously to compound (VI) from 2.0g (0.005 mole) of 2-amino-4-methyl-6-perfluoro(1-methyl-2-oxapentyl)pyrimidine (III). The yield was 1.4g (70%), yellow oily substance. IR spectrum, thin layer (cm-1): 3200,3180, 1680,1620,1540,1440,1300-1280. 2,2'-Azobis[4-methyl-6-perfluoro(1,4-dimethyl-2.5dioxaoctyl)pyrimidine (IX). It was synthesized similarly to compound (VI) from 2.2g (0.004 mole) of 2-amino-4-methyl-6-perfluoro(1,4-dimethyl-2,5-dioxaoctyl)pyrimidine (IV). The yield was 1.4g (65%), light brown oily substance. IR spectrum, thin layer (cm-1): 3390,3120,2910,2850,1680,1590,1440,1340-980. 2,2'-Azobis[4-methyl-6-perfluoro(1,4,7-trimethyl-2,5,8-triundecanyl)pyrimidine] (X). It was synthesized similarly to compound (VI) from 7.3g (0.005 mole) of 2-amino-4-methyl-6-perfluoro(1,4,7-trimethyl-2,5,8-trioxaundecanyl)pyrimidine (V). The yield was 4.47 (60%), oily substance of light-brown color. IR spectrum, thin layer (cm-1): 3392,3110,2900,1769,1683,1405, 1309-995. References:
|
Fluorine Notes, 1999, 5, 3-4