Fluorine Notes, 1999, 4, 9-10
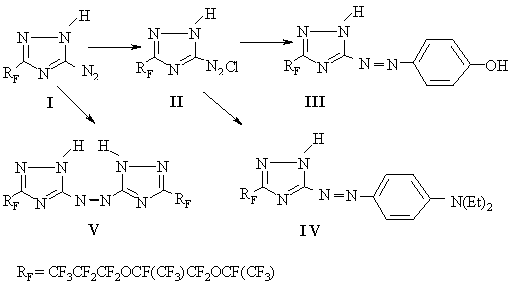
Conversions of 3-perfluoro(1,4-dimethyl-2,5-dioxaoctyl)-5-amino-1,2,4-triazole in reactions of nitrogen coupling and oxidation .S.V. Vershilov, L.M. Popova Saint-Petersburg State Technological Institute, Moskovsky ave.26 , Saint-Petersburg, 198013, Russia Diazotization of 3-perfluoro(1,4-dimethyl-2,5-dioxaoctyl)-5-amino-1,2,4-triazole with subsequent coupling with phenol and N,N-diethylaniline has resulted in formation of appropriate 5-azo-derivatives. Oxidation of 3-perfluoro(1,4-dimethyl-2,5-dioxaoctyl)-5-amino-1,2,4-triazole with potassium permanganate in an alkali medium has issued in the formation of 3-perfluoro-substituted 5,5'-azo-bis(1,2,4-triazole). To continue the previous works [1,2] on 3-perfluoro-substituted 1,2,4-triazoles with the purpose to find new effective surface-active substances including azo-dyes possessing high light-resistant properties, coloration and adhesion to synthetic materials, we have conducted synthesis of a number of 5-azo-derivatives of 3-perfluoro(1,4-dimethyl-2,5-dioxaoctyl) 1,2,4-triazole. The reaction of diazotization of 3-perfluoro-substituted 5-amino-1,2,4,-triazole was carried out in an alcohol medium at 5-10oC. The product of diazotization is stable in solutions (in an alcohol or 1,1,2-trichloroethane). The formed diazo-compound (II) was determined to enter a coupling reaction with aromatic alcohols and amines similarly to aromatic diazo- compounds [3,4]. 1,2,4,-Triazolyl-5- diazonium salt by nitrogen coupling with phenol in an alkali medium (pH 9) and by nitrogen coupling with diethylamine in a weakly acid medium (pH5) forms the appropriate azo-derivatives (III, IV) (scheme).
The reaction was carried out at an equimolar ratio of the components at 10-15oC during 3-4 hours. The yield of goal compounds (III) and (IV) was 54% and 62% accordingly. The products of azo coupling after their extraction from the reaction mixture by 1,1,2-trifluorotrichloroethane (R-113), distillation of solvent and drying under vacuum were separated as an oily yellow liquid (III) and as an amorphous substance of orange color. The structure and composition of compounds (III, IV) were confirmed by the data of elementary analysis, IR and UV- spectrometry. IR spectra of the products of nitrogen coupling (III-V) exhibit absorption bands corresponding to valence vibrations of methyl and hydroxyl groups in the range of 3505-1410cm-1, vibrations of triazole and benzene rings of 1745-1410cm-1 and C-F valence vibrations at 1350-980cm-1.
In UV spectra of azo-derivatives of triazole (III-V) there were observed
typical absorption maxima in a range of 198-210nm (lg T o evaluate stability of fluorine containing 5-aminotriazoles to the effect of oxidizers was of practical interest. So, for example, oxidizing of 3-perfluoro-substituted 5-amino-1,2,4-triazole (I) with potassium permanganate in a 10% water solution of potassium hydroxide at room temperature for 6 hours resulted in formation of 5,5'-azo-bis-derivative (V) yielded in 65% (scheme). Influence of an alkali in the absence of an oxidizer did not change the structure of 3-perfluorinated 5-amino-1,2,4-triazole (I), but only resulted in formation of the appropriate salt. The composition and structure of compound (V) was confirmed by elementary analysis and spectral data. Thus, 3-perfluoro-substituted 5-amino-1,2,4-triazoles are valuable products for further conversions in connection with a possibility to form diazonium salts ( stable in solutions). In particular, by diazo-group substitution various 5-substituted derivatives may be produced [3,5]. Another perspective direction is synthesis of azo dyes [6] which will possess surface-active properties due to the presence of the perfluorinated substituent. Experimental IR spectra were recorded on a Shimadzu IR-470 (Japan) instrument (a thin film or a suspension in Vaseline or perfluorinated oil), UV spectra were recorded on a SF-48 spectrophotometer at a concentration of 10-4 mole/L, the absorption layer thickness was 1 cm. The control over the reaction course and purity of the compounds produced was performed by a method of thin-layer chromatography on Sulifol UV-354 plates. The starting 3-perfluoro(1,4-dimethyl-2,5-dioxaoctyl)-5-amino-1,2,4-triazole (I) was synthesized according to method [1,2]. 3-Perfluoro(1,4-dimethyl-2,5-dioxaoctyl)-1,2,4-triazolyl-5-diazonium chloride (II). 30 ml of 10% solution of HCl was added to a solution of 10.7g (0.02 mole) of 5-amino-3-perfluoro(1,4-dimethyl-2,5-dioxaoctyl)-1,2,4-triazole (I) in 50 mL of 1,2,2,-trifluorotrichloroethane (R-113) and ethanol (1:1). Then a solution of 1.4g (0.02 mole) of NaNO2 in 10mL of water was added dropwise at 5-10oC and was kept for 5 hours. The product was extracted with R-113, washed with water to the neutral reaction. The solution produced was used to carry out the reaction of azo coupling. 5-n-Hydroxyphenyl-3-perfluoro(11,4-dimethyl-2,5-dioxaoctyl)-1,2,4-triazole (III). A solution of compound (II) was added at stirring dropwise at 10-15oC for 3 hours to a mixture of 1.9g(0.02 mole) of phenol and 1.7g(0.03mole) of KOH in 30 mL of water. The mixture produced was then acidified with a 10% HCl solution to pH 4-5, extracted with R-113, washed with water to the neutral reaction, dried and the solvent was distilled. The yield was 6.9g(54%), oily liquid of yellow color. IR spectrum, thin layer(cm-1): 3300-3150, 2970, 1745, 1680, 1605, 1545, 1465, 1410, 1330-980. UV spectrum (i-PrOH), lmax (nm)(lg e ): 201(3.74), 230(shoulder). C16H6F17N5O3. It was found, %: C 30.42; N10.83. It was calculated, %: C 30.06, N 10.96. 5-n-Diethylaminophenyl-azo-3-perfluoro(1,4-dimethyl-2,5-dioxaoctyl-1,2,4-triazole (IV). A solution of compound (II) at 10-15oC was added at stirring to a mixture of 3.0g(0.02 mole) of N,N-diethylaniline and 3 mL of HCl (strong) in 50 mL of 50% aqueous alcohol solution and was kept for 4 hours. The product of nitrogen coupling was separated from the reaction mass by addition of 20% NaCl solution, dissolved in 20 mL of R-113 and washed with water to pH 7, dried and the solvent was distilled. The yield was 8.6 g (62%), amorphous substance of orange color. IR spectrum thin layer(cm-1): 3160, 2940, 2180, 1740, 1675, 1605, 1550, 1465, 1410, 1350-1000. UV spectrum (i-PrOH), lmax (nm)(lg e ): 200(3.83), 234(3.66).C20H15F17N6O2. It was found, %: C 34.77; N12.18. It was calculated, %: C 34.60, N12.10. 5.5'-Azo-bis[3-perfluoro(1,4-dimethyl-2,5-dioxaoctyl)-1,2,4-triazole] (V). 5.6g (0.1 mole) of a solution of KOH in 20 mL of water was added dropwise to a solution of 12.0g (0.025 mole) of 3-perfluoro(1,4-dimethyl-2,5-dioxaoctyl)-5-amino-1,2,4-triazole (I) in 10 mL of ethanol. Then at intensive stirring a solution of 0.63g (0.025 mole) of KmnO4 in 30 mL of water was added and kept for 4 hours. The reaction mass was treated with 5% HCl solution to pH4-5. The product was extracted with R-113, washed with water, dried, the solvent was distilled. The yield was 7.8g (65%), colorless crystals, melting point of 89-91oC. IR spectrum, a solution in R-113(cm-1): 3505, 3225, 3125, 2995, 1734, 1670, 1647, 1600,1540, 1459. UV spectrum (i-PrOH), lmax (nm)(lg e ): 198(3.88), 245(3.68). Thin-layer chromatography (acetone-water, 1:1), Rf 0.37. C20H2F34N8O4. It was found, %: C 22.46, H 0.28,F 60.59, N10.38. It was calculated, %: C 22.58, H 0.18, F 60.70, N10.53. References
b). Patent 286.971 EP (1988), Bayer A.G. c). Patent 90,295 (1986) Roumania |
Fluorine Notes, 1999, 4, 9-10