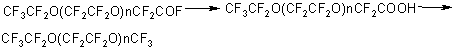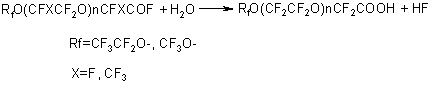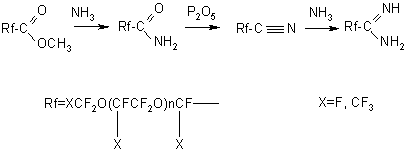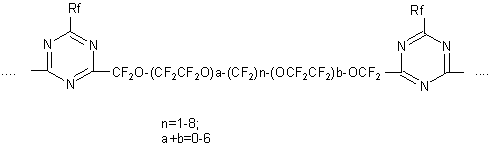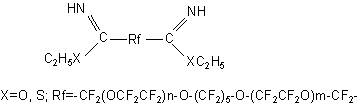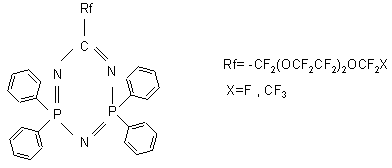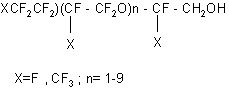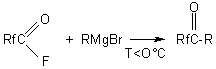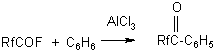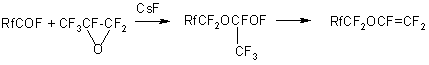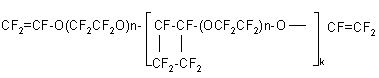Fluorine Notes, 1999, 3, 5-6
Tetrafluoroethylene oxide and its derivativesReport 3. Properties and application of tetrafluoroethylene oxide derivatives A high and steady interest of researchers and experts in organofluorine compounds is explained by a great progress in chemistry of organofluorine compounds and, on the other hand, by extremely valuable characteristics of articles produced ( high thermal and frost resistance, chemical stability to different aggressive compounds, etc.). Derivatives of tetrafluoroethylene oxide (TFEO) are distinguished among other fluoropolymers by a complex of attractive properties caused by high strength of the C-F, C-C and C-O bonds, flexibility of chains and a small value of the interaction forces between the chains. The specific properties of heterochain fluoropolymers are to a great degree associated with a specific nature of the fluorine atom and its bonds with carbon in these compounds. The highest electronegativity (4) among the elements of the periodic system and van-der-Waals radius comparable with that of hydrogen (1.25A in comparison with 1.1A for hydrogen) cause a high energy of the C-F bond and comparatively small differences in steric interactions of fluorine atoms in comparison with hydrogen atoms in compounds of similar structure: the radii of fluorine and hydrogen atoms are less than the lengths of the C-C bond. Thus, replacement of hydrogen atoms with fluorine atoms in the mentioned polymers does not bring any noticeable steric limitations at conformational transitions despite a significant change in bond electron characteristics. This specific nature of the C-F bonds allows to use the fluorine atoms in natural polymers for a directed change of their properties without a significant disturbance of stereochemical interactions causing a wide variety of functions of biological systems (2,3). In the report presented, properties of oligomers based on TFEO depending on the way of their production and also application of TFEO oligomers, products of their stabilization and functional derivatives on their base are under consideration. Properties and application of TFEO oligomers. The properties of TFEO oligomers depend to a great extent on the synthesis conditions. In solid phase polymerization, a chain growth of the crystal lattice is mostly influenced , consequently polymers with greatest molecular mass are formed (4). Such polymers possess thermal resistance even higher than fluoroplast: the activation energy of thermal destruction is 98 and 86 kcal/mol and maximum decomposition rate is attained at a temperature of 628oC and 568oC respectively. Investigations of fluoropolymers produced under ionization radiation have shown, that their molecular mass is 100, 000-170,000, they do not contain functional end groups and possess the crystal structure with a partial macromolecular orientation. Such a product is a sole representative of high-molecular perfluoropolyethers and is remarkable for extremely high chemical and thermal stability (6). TFEO oligomers of comparatively high molecular mass have been produced on activated carbon (7), some of their properties are given in the Table below:
Production of liquid TFEO oligomers has been described in details in patents (8,9). In this case a mixture of oligomers of the general formula CF 3 -CF2 -O(CF2 CF2 O)n-CF2 -C(O)Fwith a polymerization degree of up to 50 is formed. The boiling point of TFEO oligomers with a polymerization degree up to 10 is given in the Table below:
In the photooxidation of TFE in gas phase a mixture of oligomers of the general formula CF3O-(CF2O)n - (CF2CF2O)m - C(O)F is formed at n=12 and a ratio of n/m=10(10). Polymers of a similar structure were produced also in TFE oxidation by ozone, in this case a number of polyether units -CF 2 CF2 O was in proportion to the TFE pressure in the reaction mixture (11). The molecular mass of the products produced attained 5,000 – 15,000, that was slightly higher than the molecular mass of the polymers produced by TFE photooxidation (1500).To use TFEO oligomers as chemically and thermally stable liquids and oils, the reactive fluoroanhydride groups are replaced with more stable ones (1,12-14). Thus, inert polyfluoropolyethers with enhanced thermal stability are produced by fluoro- anhydride group replacement with the fluorine atom. For that, perfluoropolyoxaacyl fluorides are transformed to acids, then treated with fluorine at a temperature of 120-250oC (15,16). As a result of decarboxylation and fluorination, inert products of the following structure
are produced. The replacement of fluoroanhydride group with the fluorine atom can be executed also by fluorination at a reduced temperature (50-350oC) in the presence of catalyst (magnesium, aluminum, zinc, lead, bismuth etc.) (17). Heating perfluoropolyoxaethylencarbonic acids in an aqueous medium in the presence of potassium persulfate at a temperature of 50-100oC results in the formation of perfluorinated ethers (18):
The end fluoroanhydride group can be replaced with a hydrogen atom by heating an alkali metal salt of the appropriate perfluoropolyoxaethylencarbonic acid in ethylene glycol (19):
An exposure of TFEO oligomers to UV-rays ( the wave length from 2500 to 3700A) in a nitrogen medium at a temperature from –80oC to 200oC results in the detachment of the fluoroanhydryde group and the formation of inert perfluoropolyethers with the doubled molecular weight(20):
The same result is attained using plasma instead of UV-irradiation. Thus, placing perfluoropolyoxaacyl fluoride in a plasma furnace at a residual pressure of 1-3 mm of mercury column at a temperature of decarboxylation leads to the formation of perfluoropolyether with the twofold weight in a yield up to 94% (21). A set of various bond characteristics in polyether molecules together with inertness of the end trifluoromethyl groups determine high thermal and thermal oxidation stability, low glass transition temperature, exceptional resistance to strong oxidizers and many other unique properties of stabilized perfluoropolyethers based on TFEO (1, 22-24). A consequence of chemical resistance of perfluoropolyethers is their incombustibility and biological inertness (22,25). Polyethers stabilized by one of the mentioned methods ( fluorination, irradiation, alkali treatment ) can be applied as high-temperature motor oils, hydraulic liquids, heat carriers, high stable plastic lubricants and etc. (1,20,28-39). More over, TFEO oligomers are applied for modification of surface properties of polymeric materials to give them oil-, water-, mud proof properties (18). Synthesis and properties of TFEO oligomer derivatives containing various functional groups. The fluoroanhydryde group of TFEO oligomers is easy to transform by known chemical conversions to other functional groupings depending on the direction of further application of the perfluoropolyether (1,12,13). Thus, treatment with water transforms TFEO oligomers into the appropriate perfluoropolyoxaethylencarbonic acids:
Hydrolysis of fluoroanhydrides is carried out either directly by water or by aqueous solutions of salts (CaCl) or alkalis followed by acidation of the hydrolysis products with hydrochloric acid (7,16,40-41). There is no data in literature on the direct application of perfluoropolyetheric acids based on TFEO, as a rule, they serve to produce other derivatives. Different salts are easy to form from perfluoropolyoxaethylencarbonic acids and from TFEO oligomers having the end fluoroanhydride groups. Salts of alkali metals, ammonia and substituted amines are recommended to use as surface-active substances and dispersing agents (7,42). A treatment of perfluoropolyoxaammonium carbonic acids or acylfluorides with alcohols results in the appropriate esters (8,43). Methyl esters of perfluoroetheric acids are easy to saponify with alkalis to form salts. Mainly, methyl esters are used as intermediate products for synthesis of other derivatives. Starting from methyl esters of perfluoroacids, the appropriate amides, nitriles and amidines are formed (43):
Similarly dinitriles based on TFEO copolymers and difluoroanhydride of glutaric acid were produced with the following structure (23,43-45): NC-CF2-(OCF2CF2)n-O-(CF2)5-O-(CF2CF2)mCF2-CN; NC-(CF2)4-O-(CF2CF2O)pCF2-CN The dinitriles produced are the starting compounds for synthesis of polyperfluorotriazine polymers (43,45):
The presence of hinged oxygen atoms in the main chain of triazine polymers essentially improves their low-temperature elastic properties and slightly increase their heat resistance (43). Besides, interaction of dinitriles based on TFEO oligomers with alcohols or thiols results in the formation of compounds of the following formula:
used as monomers in the synthesis of polymers resistant to oxidation and hydrolysis (23,44). Diphosphatriazines of the formula
are used as additives, corrosion inhibitors, to oils and lubricants based on Krytox perfluoropolyethers (46). Reduction of perfluoropolyoxaacylfluorides by boron hydride of an alkali metal in an inert solvent (dioxane) leads to the formation of the appropriate perfluoropolyether alcohols of the formula (9,47):
Interaction of the perfluoropolyether alcohols of the mentioned type with pyromellitic anhydride or cyanuric chloride is used for production of lubricants possessing high thermal stability (8). Synthesized diethers of phosphoric acid on the basis of perfluoropolyetheric alcohols of the formula: XCF2CF2O(CFXCF2O)nCFXCH2O]2PO (OM) X=F,CF3 ; n=1-8; M = H, Li, K, Na, NH4 possess good oil-resistant properties (9). Thus, on the basis of TFEO oligomers, alcohols of the formula: CF3O(CF2CF2O)nCF2CH2OH , n =1-5 were produced. They are oilproof and have a low glass transition temperature:
The produced alcohols can be used in chemical industry in synthetic rubber industry to produce oilproof acrylates with a high frost-resistance that as latexes are used for finishing textile materials. In the reaction of hydrocarbon and fluorocarbon Grignard agents with fluoroanhydrides of perfluoropolyoxaethylencarbonic acids, fluoroketones were produced (48):
Rf = C2F5O(CF2CF2O)nCF2- ; n=0-2 R=C2H5,C6H5 ,C6F5 ,C6F4Br , C6F4H Analogous aryl-perfluoropolyoxaalkylketones were produced by benzene acylation according to Friedel-Crafts (48):
Rf =C2F5O(CF2CF2O)4CF2 - Perfluoropolyoxaalkyl-substituted arylphosphines based on TFEO oligomers of the general formula:
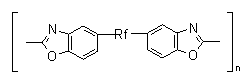
find an application as an anticorrosive and antioxidant additives to perfluorinated liquids (49). Heat-resistant elastomeric polybenzoxazones having polyperfluoroalkylenoxide bridges in the chain are used as sealants for cosmetics (50):
Rf=-CF2 (OCF2 CF2 )n-O-(CF2 )5 -O-(CF2 CF2 O)m-CF2 - Perfluorophenylalkylenoxadiacetylenes of the formula:
produced on the basis of the co-polymer of TFEO and difluoroanhydride of 2-oxaglutaric acid
are used as monomers for producing elastomeric perfluoropolyethers (51). The ammonium forms of fluoropolymers: -(CF2CF2)p{CF2CF[O(CF2CXFO)e]m(CF2)nCH2N+R1R2R3*Z}q, x=F,Cl,CF3 ; e =0 - 5 ; m= 0 - 1; n=1 - 5; p/q = 2 -16; z = Cl-, SO42- are used as chemically stable anionites or anion-exchange membranes in electrolyzers (52). TFEO oligomers are used for modification of the surface of polymer materials to give them water- and oil-resistant properties (53) ( styrene, butadiene, isoprene, methyl methacrylate etc.). Block copolymers based on TFEO and vinyl monomers possess an improved resistance to organic solvents, elevated resistance to impact and low gas permeability (54,55). They can be processed into films, threads, pipes, molded articles, foam structures, nonwoven fibers. An important property of TFEO oligomers is their ability to add HFPO resulting in the formation of perfluorinated alkylvinyl ethers via pyrolysis, for example (56,57):
Rf =CF3 CF2 O(CF2 CF2 O)nCF2 - Fluoroanhydride of perfluoropolyether acid is either directly subjected to pyrolysis at a temperature from 300 to 600oC in the presence of sodium sulfate (56) or metal oxides (57) as a catalyst , or the fluoroanhydride is preliminarily transformed into a salt of alkali metal and subjected to pyrolysis as a salt at a temperature of 170-250 oC. In the presence of a polar solvent (diglyme), pyrolysis can be carried out at temperatures below 100oC. Perfluorinated alkylvinyl ethers based on TFEO oligomers are easy to copolymerize with a number of fluoroolefins, for example, with TFE to form fluororubber (22,45) or modified polytetrafluoroethylene which can be processed by pressure moulding and extrusion (57). An introduction of ether groups into fluorocarbon polymers significantly reduces rigidity of polymer chains, that leads to changes in a number of physical properties associated with flexibility of macromolecules: especially a signicant reduction of the glass transition temperature; in this case the thermal stability of the fluoropolymers remains approximately the same and lies within a range of 250-300oC (45). In pyrolysis of fluoroanhydrides of difunctional perfluoropolyetheric acids, the appropriate diperfluorovinyl ethers are formed (58) CF2 =CF-(OCF2 CF2 )n-O-(CF2 )m -O- (CF2 CF2 O)c - CF=CF2 On the basis of divinyl ethers, cross-linked polymers of the following structure are synthesized (58):
These polymers are thermally stable up to 300oC and resistant to exposure of boiling sulfuric and nitric acids and also to 20% aqueous solution of KOH. The heating of divinyl ethers of the following type: CF2 =CF-O-(CF2CF2O)n-CF=CF2 in inert medium at a temperature of 100-200oC and a pressure of 1000-5000 atm results in the formation of perfluoropolyethrs containing perfluorocyclobutane units in the main chain, for example (59):
n=2-20 , k=1-10 Further radical polymerization of the produced oligomers leads to the formation of thermoreactive resins with a high cross-linking degree. Vinyl ethers on the basis of TFEO oligomers have found application in synthesis of heat- and frost-resistant rubbers. Copolymerization of vinyl ethers of the formula: CF3 O(CF2 CF2 O)nCF=CF2 with vinyliden fluoride and other fluorine-containing olefins was used to produce , for example, heat resistant, chemically inert high-molecular curable copolymers with a glass transition point of minus 80oC. Vulcanized rubbers, produced on the basis of such co-polymers, possess thermal resistance in strain state and may be used as layings, membranes, and other articles under conditions of a wide range including frost resistance down to –50-60oC, heat resistance up to 250oC, chemical stability towards acids and alkalis, oil- and gasoline resistance. Unsaturated fluorine-containing ethers based on TFEO oligomers can be also produced by dehydrohalogenation or dehalogenation of ethers of the formula (60,61): RACF2O(CF2CF2O)nCF2COR', R= F, SO2F, COF, COOH, CONH2 ; R'=F,OH,Oalk,OM etc. n=0-50 and A is fluorine containing alkylen containing 2-5 carbon atoms. The unsaturated fluorine containing ethers produced are intermediate products of synthesis of ion-exchange membranes, surfactants, emulsifiers, solvents, agents for purification of fibers or metals. Conclusions. In a brief report it is impossible to describe in detail and completely all derivatives of tetrafluoroethylene oxide such as, for example, polyperfluorotetramethylene oxide, bifunctional derivatives. Moreover, due to a number of reasons, we could not include some papers of our native researchers such as F.M.Moukhametshin, I.P.Kolenko, N. A. Riabinin, V.A.Soshin, Ja.M.Vilentchik, G.I.Lekontseva and many others. The main task of this paper was to draw attention of researchers and experts to a possibility to create novel advanced materials based on TFEO derivatives and possessing unique properties. If this paper has conveyed the idea of the existence of the extendable horizons for TFEO applications, we consider our aim achieved. REFERENCES 2."Fluoropolimers", edited by Leo A. Wall. Willey, Interscience,1972. 3. R.E. Banks, D.M. Birchell, R.N. Hazeldin. Khimiya i tekhnologiya polimerov, N 3, 133 (1961) 4. Barnaba P., Cordischi D., Lenai M., Mele A. Polimerizzatione dell`ossido di tetrafluoroetilene.- -Chem.e.Ind. (Ital.), 1965, v.47, N10, p.1060- 1063 5. A.Donato, M.Zenzi, A.Mele, Y.Macromol.Zci.Chem.A1(3), 429 (1967). 6. US Patent 3355397, Tetrafluoroethylene epoxide polyethers /Warnell J.L., 28.11.68. 7. US Patent 3125599, Polymer of Fluorocarbon Epoxides /Warnell J.L., 17.03.64. 8. US Patent 3250806, Fluorocarbonether of Tetrafluoroetheylene epoxide /Warnell J.L., 10.05.66. 9. US Patent 3293306, Perfluorinated Ether Alkohols /Li Bleu R.E., Fassnacht J.H. 22.12.66. -C.A., 1967, 66, 104686g. 10. D.Sianesi, G.Bernordi, G.Moggi, IIEuropian Simposium of Fluorine Chemistry.Gottiugen, 1968. 11. F.Gozzo, A.Bergoin, G.Camaggi, V.Opraudi.Europ. Polimer J.6, 219 (1970). 12. Eleuterio H.S. Polymerization of Perfluoro Epoxides.- J.Macromol.Sci.-Chem., 1972, A6, N6, p.-1027-1052. 13. Tarratset P., Stami E. Okisi perftorolefinov.-ZhVKhO im. D.I. Mendeleeva, 1970, t. 15,v. 1, s. 34-44. 14. Mamchenko B.F. Ftorsoderzhashchie politioehfiry i poliehfiry. Uspekhi khimii, 1971, t. 40, N 3, s. 547. 15. US Patent 3242218, Process for preparing Fluorocarbon Polyethers. /Miller W.T., 22.03.66. 16. UK Patent 1000802, Polymers of perfluoro-olefin epoxides /Du Pont de Nemours and Co., 11.08.65-С.А., 1965, v.62, 9313g. 17. Patent appl. DE 2451493, Verfahren zur Herstellung von perfluorirten A`thern. /Halasz S.-P., Kleuge F., 6.05.76. 18.Patentappl.JP60-31535,18.02.85. 19.US Patent 3342875, Hidrogen capped fluorocarbon polyethers. /Selman S., Smith W.S., 19.09.67. 20. US Patent 3214478, Perfluoroolefin epoxide polyethers /Milian A.S., 26.10.64. 21. Patent appl. JP 57-70826, 01.05.82;С.А.1982, 97, 917670. 22. Sokolov L.F.,Valov P.I., Sokolov S.V. Poluchenie monomerov dlya sinteza ftoruglerodnyh polimerov okisleniem ftorolefinov.-Uspekhi khimii, 1984, t. 53, N 7, s. 1222-1246. 23. Men'shov V.M., Kiselyova L.A., Ponomarenko A.V., Mashinskaya S.G. Konformatsionnaya statistika sverhgibkih tsepej polidiftormetilenoksida i politetraftorehtilenoksida.-Izv. AN SSSR, ser. khim., 1984, N 1, s. 104-112. 24. Shepard U., Sharts K. Organicheskaya khimiya ftora. M., Mir, 1972 . 25. Du Pont details chemistry of perfluorocarbon epoxides.- Chem.Eng.News, 1967, v.45, N33, p.18. 27. US Patent 4438006, Perfluorinated aliphatic polyalkylether lubricant with an additive composed of an aromatic phosphine substituted with perfluoroalkylether group./Shyder C.E., Tamborski C.;- 20.03.84. 26. US Patent 3385882, Fluoroalkyl glutarates. /Tullio V., 28.05.68. 28. Chem. Eng. News, 45, N 33, 18 (1967). 29. Chem. Eng. News, 45, N 27, 12 (1967). 30. D. Sianesi. Am. Chem. Sop. Polimer Preprinbs, 12, N 1,411 (1971). 31. D. Sianesi. Chem.e.ind.(ibal) 50, 206 (1968). 32. Y. Autonucci, S. Wall., J. Res. Nat. Bur. Stand., 71A., 33 (1967). 33. UK Patent 1038365, 1966. 34. DE Patent 1249247, 1967. 35. FR Patent 1366119, 1964. 36. US Patent 3483129 / Doll R.E. et al., 1969. 37. US Patent 3481872 / Doll R.E. et al., 1969. 38. US Patent 3505229 / Shekan Y.T., 1970. 39. US Patent 3306853 / Fogg E.T., Gumprecht W.H., 1967. 40. US Patent 3250808, Fluorocarbon Ethers Derived from hexafluoropropyleneepoxide. / Moore E.P., Milian A.S., Eleuterio H.S.;- 10.05.66. 41. US Patent 3250807, Dicarboxylic Acids of Fluorocarbon Ethers and Fluorides, Esters, Amides and Salts thereof. /Fritz C.G., Moore E.P. 10.05.66. 42. US Patent 3271341, Aqueous colloidal dispersions of polymer. /Garrison W.E., 6.09.66. 43. US Patent 3317484, Polymers having Recurring Triazine Rings. /Fritz C.G., Warnell J.L., 2.05.67. 44. US Patent 4147858, Fluorocarbonether bibenzoxazole oligomers containing reactive acetylenic terminal groups. /Evers R.C., 3.04.79. 45. Sokolov S.V., Kagan E.G., Ivanova T.L. Termostojkie elastomery. ZhVKhO im. D.I. Mendeleeva, 1974, t.19, v. 6, s. 650-661. 46. US Patent 4194983, Perfluorinated polyalkylether based on lubricant composition. /Paciorek K.I.L., Kratzer R.H., Kaufman J.,-25.03.80. 47. US Patent 3492374, Polyfluoropolyoxa-alkylphosphates. /Li Bleu R.E., Fassnacht JJ.H., 27.01.70. 48. Gopal H., Tamborski C. Fluoro-Ketones. II. Reactions of fluorocarbon grignardes and copper compounds with perfluoroalkylacid fluorides. / J.Fluor.Chem. 1979, v. 13, N14, p.337. 49. US Patent 4011267, Perfluoroalkylether substituted aryl phosphines and their synthesis. /Tamborski C., Snyder C.E., 8.03.77. 50. US Patent 3994861, Long-chain-perfluoroalkylene ether bibenzoxazole polyymers. 51. US Patent 4241223, F-Phenylalkylene oxide diacetylenes. /Baucom K.B., 23.12.80. 52. Patent appl. JP 60-84313,13.05.85. 53. Patent appl. JP 60-31535, 18.02.85. 54. Mamchenko B.F. Ftorsoderzhashchie politioehfiry i poliehfiry. Uspekhi khimii, 1971, t. 40, N 3, s. 547. 55. US Patent 3114778 Fluorinated vinyl ethers and their preparation / Fritz C.G., Moore E.P., 17.12.63. 56. US Patent 3274295 Block copolymers of vinylidene compounds and perfluorinated nonvinyl monomers. /Baker W.P., 20.09.66. 57. US Patent 3321532 Fluorocarbon ethers. /Lorenz C.E., 23.05.67. 58. Ponomarenko V.A., Krukovskij S.P., Alybina A.Yu. Ftorsoderzhashchie geterotsepnye polimery.-M. Nauka, 1973, s. 50-146. 59. US Patent 3397191 Fluorocarbon ethers. /Beckerbauer R., 13.08.68. 60. Patent appl. JP 54-135722, 22.10.79. 61. Patent appl. JP 54-135723, 22.10.79.
|
Fluorine Notes, 1999, 3, 5-6