Fluorine Notes, 1999, 3, 3-4

Vsevolod V. Berenblit,
Ph.D., Leading Scientist of Laboratory of Fluorganic Polymers.
S.V. Lebedev Synthetic Rubber Research Institute.
Electrochemical fluorination of organic compounds.
3. Anode process nature in ECF.
In the first publications devoted to ECF, Simons[9] has shown that the process takes place without
elemental fluorine evolution. The fluorine evolution begins only at an electrode voltage
above 7-8v, that leads to the full destruction of the organic substrate to be fluorinated
and accompanied with explosions in the electrolyzer. Nevertheless authors [32] postulate
that the process takes place by means of fluorine ion discharge on the anode and secondary
interactions of the fluorine radical with an organic molecule. Similar ideas were developed
by Okatov [40] Knuniants and Sokolsky [41] who assumed even fluorine radical diffusion into
the electrolyte mass. To explain an induction period prior to the beginning of organofluorine products evolution, Haszeldine
et al. [42] in their study of the electrochemical fluorination of sulfur-containing organic
compounds assumed that the fluorination was carried out through the forming nickel fluorides
on the anode. Then the mechanism of ECF is reduced to electron transfer by a higher metal
fluoride similar to the fluorination by cobalt trifluoride [4]. But special investigations
of behavior of different anode materials in ECF [43] have shown that this process does not
occur on cobalt and (being only of anode specific) takes place only on nickel and to a lesser
degree on its alloys. The nickel electron structure suggests the presence of nickel dioxide
in alkali medium, which is the basis of alkali batteries, but compounds of oxidized nickel
in an acid medium are not known [2]. But when a mixture of nickel chlorides and potassium
chlorides was treated with fluorine at heating, crystal structures of hexafluoronickelates
of heavy alkali metals were produced and studied[44]. Since the organic compounds exist in AHF medium as protonated cations, Burdon and Tatlow [45]
and later others [46,47] assumed that the ECF process occurs with participation of complex
higher nickel fluorides in which the nickel of the highest valence acts as an anion complexing
center, fluorine atoms act as ligands and the protonated organic compound acts as a cation
according to the scheme: 2B:H+ + Ni + 6F- -4e- —->(B:H)2NiF6
One can assume a possibility of intramolecular transfer of electrons in this complex and, as
a result of this, nickel passes into 2-valent state and fluorine replaces hydrogen
in the organic compound with HF evolution. Rozhkov [11] and Schmidt [2] considered the mechanism of carbcation formation in electrochemical
oxidation of an organic molecule and an addition of fluorine anion proved for the fluorination
on Pt anode in a medium of acetonitrile and acetic acid. They made an attempt to generalize
this mechanism with that of electrochemical fluorination in AHF medium. But it seems not
productive to prove the unified mechanism in such different media and on anodes of different
structure. As it was mentioned, the simplest process of electrochemical fluorination is water fluorination
with formation of fluorine monoxide. Hygroscopicity of AHF and moisture condensation on a
low temperature reflux condenser of the electrolyzer signify that moisture is almost always
present at the beginning of ECF. During the elctrolysis water is fluorinating and according
to author [9] its content in the electrolyte after some hours does not exceed 0.1%, while
the effectiveness of the process comes down significantly when water content increases to
1% and at a value of 10% there is the formation of explosive mixtures in the electrolyzer.
According to Simons [48], it is possible to produce perfluorooctane in 30% yield using water
conductive properties in the octane electrolysis in the presence of 1.5% of water. In this connection the mechanism of water oxidation in AHF and water influence on the ECF process
have been a subject of researchers' steady attention. In particular Engelbrecht [49] studied
the water electrolysis within a wide range of concentrations and has shown that at a moisture
content above 30%, the only anodic process is water discharge with formation of oxygen and
ozone. As the concentration of water decreases, the oxydation and fluorination start to compete
and at a concentration below 20% there is found up to 60% of fluorine monoxide. Rogers et
al. [50] studied hydrogen fluoride drying by electrolysis and have shown that the current
drop in the elctrolysis of moist hydrogen fluoride is determined not only by the moisture
content reduction but also by a change of the anode condition during the electrolysis, so
full drying can be achieved only using a reverse current. Nagase et al. [51] studied water
effect on the process of electrochemical fluorination of alcohols and esters of carboxylic
acids. It has been determined that the presence of moisture in amount of 0.2-0.7% in the
elctrolyte affects negligibly the perfluoroacyl fluoride yield, but the current yield of
these products decreases significantly though moisture removal was made earlier
than fluorination of the main substrate. Different authors tried to prove their assumptions of the fluorination mechanism studying the
content of the fluorination products but strong arguments of the nature and mechanism of
the electrochemical process could be obtained only using modern electrochemical methods of
investigation of electrode processes. Their realization has been limited for a long time by a possibility to measure the anodic potential
in AHF versus a stable nonpolarizable reference electrode. For the first time Fredenhagen [52] measured in AHF the electrode potential difference of gas
electrodes (hydrogen and fluorine) on platinum and determined experimentally the standard
fluorine potential equal to 2.76V at 0oC. Koerber and De Vries [53] have measured the equilibrium electrode potentials of metal fluoride
electrodes in AHF versus mercurous fluoride electrode, they have determined EMF for chains
without migration of the following types: M(Hg)|MF2(s)||HF(NaF)||Hg2F2||Hg
where M=Cd; Cu, and also Pb(Hg)|PbF22.5HF(s)||HF(NaF)||Hg2F2|Hg
and for chains with migration of Hg|Hg2F2||HF(NaF)||HF(NaF;H3OF;AgF)||Ag
type. The authors showed that in AHF medium it is possible to determine emf with an accuracy
of 0.1 mV and the concentration dependencies of the electrode potentials are in accordance
with Nernst’s equation. It was shown that lead fluoride in this medium formed an insoluble
complex, also free energies of formation of metal fluorides and the values of the activity
coefficients of silver fluoride solutions in AHF were determined. Some attempts [54,55] were
made on a polarographic investigation of ECF but they were of little information. Hackerman et al. [56] first have used a mercurous fluoride reference electrode
in polarization measurements in AHF medium whereas Kaurova [59] and Watanabe [57,58] used
a hydrogen reference electrode. The apparatus illustrated in
Fig.3
is that designed by Watanabe, the cell of Kaurova's design is illustrated in
Fig.4
. Later Burrows [60] and Novak [61] developed for this purpose a cupric fluoride electrode
and showed that the latter is the most convenient and stable reference electrode in this
medium. Using water as a fluorination model, Hackerman and co-workers made potentiostatic and halvanostatic
investigations of anodic processes and measured the differential capacity of the double layer
in the anhydrous and moist hydrogen fluoride. They showed with evidence that metal dissolution
with formation of a passive film of nickel fluoride takes place on nickel in the anodic range
of the potentials. When the potential reaches a value of fluorine evolution, the anode current,
according to Tafel's equation at a slant b equal to 600mV, conforms to the only
process within the range of high anodic potentials which is the process of fluoroanion discharge
with formation of fluorine radical which being adsorbed in the nickel fluoride film, begins
to form molecules with elemental fluorine evolution only at potentials above 5.5V. These
ideas were confirmed both by electrochemical measurements in AHF in the presence of moisture
[57,59] and by the study of fluorine absorption on nickel fluoride [58]. There was determined
the energy of fluorine absorption on nickel fluoride within the range of temperatures of
(-72 +27oC), its value (below 4 kJ/mol) together with the slope of Tafel's curve
are an evidence that the limiting stage of the process is fluorine ion discharge. This research
has confirmed the extremely high value of b and hence a value of the electron transfer
coefficient Serushkin and co-workers [63] studied charging curves on the nickel anode in AHF in the presence
of moisture and acetic acid. Considering the eletricity amount corresponding to the delays
on the curves, they determined that the filling of the nickel surface is equal to 1
10-8 mol/cm2 which is in conformity with formation of 3-4 layers of
nickel fluoride on the anode. But a difference between the charging curves in solutions of
acetis acid and water do not point to a change of the anode process nature. In the presence of moisture, anodic nickel dissolution comes down significantly, the current
yield of the corrosion process and passivation potential decrease, all this is associated
by the authors with complexation of nickel fluoride with water ( Clifford [64]). In dependence
on the moisture content, nickel fluoride exists in this medium as aquacomplexes: dehydrate,
tetrahydrate and hexahydrate. Water which is protonated in this medium and exists as a hydroxonium
cation, at interaction with nickel fluoride is subjected to deprotonation. Earlier there
was found complexation of various organic compounds with metal fluorides [65], especially
with nickel fluoride which complexation with acetonitrile was studied quantitatively and
there was found the formation of insoluble solid phase of Ni(CH3CN)nF2 at increasing the acetonitrile concentration up to 1M. Complexation of nickel fluoride with organic compounds accompanying their deprotonation on the
anode surface obviously removes the electric barrier preventing cation participation in the
anodic reaction at high potentials. It followed from the first Simons' publication [66] that a process of ECF is not selective in
most cases. It is accompanied by the following processes: 1.destruction of the carbon skeleton of the starting compound: 2.bond cleavage of carbon with a heteroatom: 3.cyclization: 4.cycle narrowing: 5.cycle opening along C-C bond: 6.isomerization of the original skeleton with formation both branched structures: and linear structures on the other hand [81]: Variety of radical processes accompanying ECF seems to confirm the concept [45-47] that this
process does not differ fundamentally from the interaction of organic compounds with fluorides
of metals of variable valence. But in fluorination of N-isoamylmorpholine the ratio of isomers
in the reaction products is inverse: 96% of isostructure and only 4% of the normal one. In the ECF process of chlorine-containing compounds, chlorine in large part is kept in the structure
[82-84] whereas in fluorination of these compounds by elemental fluorine ,chlorine is replaced
with fluorine easier than hydrogen. According to our concept, adsorption of deprotonated organic molecules on the surface of nickel
fluoride can occur not only by means of complexation. Obviously, nickel fluoride saturated
with fluoro radicals [58] gains electron conductivity and organic molecules obtain a possibility
to be adsorbed in dissociation on its surface like it takes place at adsorption of organic
compounds on platinum when unpaired electrons of the radicals interact with the electron
set of Pt [85]. Such an adsorption is accompanied with electrochemical desorption of hydrogen
radicals and appearance of radical chains on the anode surface . The chains interact with
fluoro radicals and are desorbed as perfluorinated molecules. The formation of radical centers
in the carbon chain makes possible cyclization, ring narrowing, migration of methyl group
and carbonylfluoride group along the chain that makes easier desorption of perfluorinated
compounds from the anode surface. 4. Influence of the structure of organic compounds on the
fluorination product yields. Already in the 1950-s it became clear that the structure of organic compounds in general and
functionality in particular affect considerably yields of nondestroyed products of electrochemical
fluorination. First of all a difference in the yields attracted the attention of researchers
at fluorination of various carboxylic acids , their derivatives and N-substituted compounds
to a different degree amines . Incidentally fluorination of the same compounds gave very
different results. Simons and co-workers reported in their first publications about the presence
of only traces of trifluoroacetyl fluoride in the electrolysis of acetic acid, but other
authors informed about the yields varying from 2.5 to 20% mol [40,84-90] in fluorination
of this acid. For higher carboxylic acids, C8-caprylic acid in particular, the
yields vary from 3 to 16%mol [88-91]. ![]() calculated
according to the equation for b : b =2.3 RT/(1-
calculated
according to the equation for b : b =2.3 RT/(1- ![]() )zF, which varies from 0.8 to 0.9 (according to different authors)
and points to an energy barrier distortion. This phenomenon is typical enough for the processes
in the range of high anodic potentials associated with radical absorption on the electrode
surface covered with a film of oxides as in Kolbe's synthesis [62].
)zF, which varies from 0.8 to 0.9 (according to different authors)
and points to an energy barrier distortion. This phenomenon is typical enough for the processes
in the range of high anodic potentials associated with radical absorption on the electrode
surface covered with a film of oxides as in Kolbe's synthesis [62].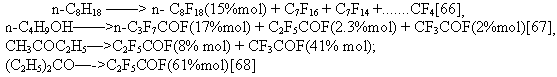
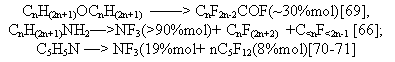
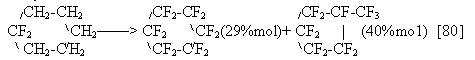
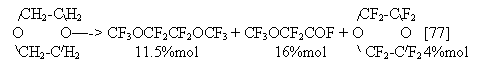
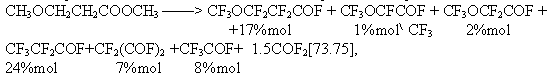
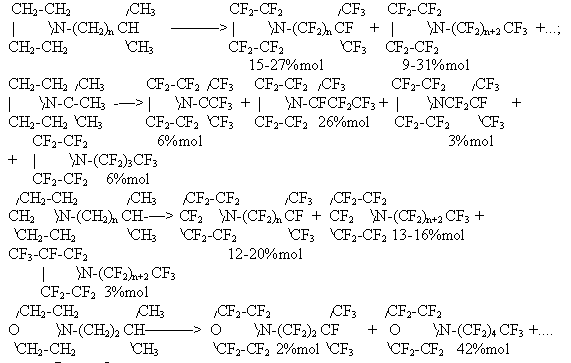
It is of interest that alkoxy group introduction into the acid structure increases considerably carboxy group stability. For comparison, under conditions of our experiment [92] in fluorination of acetic acid the yield of trifluoroacetyl fluoride did not exceed 3%mol, whereas the yield of perfluoroacyl fluorides was sighificantly higher in electrolysis of alkoxy derivatives of acetic and propionic acids:
| Starting substrate |
Yield, %mol |
|||
|
RFO(CF2)nCOF (n=1;2) |
CF3CF2 -COF |
CF3-COF |
|
|
|
CH3OCH2CO2H |
2.5 |
- |
11 |
13.5 |
|
CH3CH2OCH2CO2H |
2.0 |
- |
17 |
19 |
|
CH3(CH2)2OCH2CO2H |
2.0 |
19 |
5 |
26 |
|
CH3O(CH2)2CO2H |
8.0 |
20. |
13 |
41 |
|
CH3(CH2)2O(CH2)2CO2H |
6.0 |
10 |
12 |
28 |
The yield of trifluoroacetyl fluoride is noticeably higher in fluorination of acetic anhydride than in case of acetic acid, but it varies also from 15 to 40% mol [40, 86-90]. And at last, maximum yields of trifluoroacetyl fluoride were attained in acetyl fluoride fluorination [91]. Such a change in yields is regular enough at transition from acids to anhydrides keeping in mind the solvolysis of anhydrides:
In fluorination of acylfluorides and chlorides there were obtained other perfluoroacyl
fluorides with maximum yields [91] . An increase in the length of the acetylfluoride carbon
chain results in a yield reduction of appropriate perfluoroacylfluorides as it is shown in
Fig.5
according to data [91]. The electrochemical fluorination of alkanosulfo acids has not resulted in a noticeable
yield of perfluoroalkanosulfofluorides, whereas fluorination of methanesulfofluoride gave
a yield close to a quantitative one and its value was 96% mol [93]. Electrochemical fluorination
of alkanosulfofluorides and chlorides with a number of carbon atoms of up to 9 [93-95] allowed
to obtain perfluoroalkanosulfofluorides in acceptable yields which decreased at the chain
growth more slowly than in case of acylfluorides, that was an evidence of less destruction
of C-S bond. There were made attempts [39, 40, 45] to interpret the process of decarboxylizing in the fluorination
of carboxylic acids similarly to the well-known Kolbe’s electrosynthesis on platinum [65]
when the carboxylated anion is dicharged with formation of an adsorbed radical with its subsequent
decarboxylizing at electrochemical desorption or heterogeneous recombination: TThe following scheme was proposed [45]: RFCOO- - e- -> RFCOO’ - > R’-F +R-H which is open to criticism because there are no anions of carboxylic acids in AHF medium and
perfluorinated structures are adsorbed from the anode surface. A distinguishing feature of
Kolbe’s electrosynthesis is radical dimerization slightly observed at electrochemical fluorination
and it is difficult to consider hydrogen fluoride disproportionation under the action of
radicals. A source of unsatisfactory yields of perfluoroacylfluorides in the ECF of carboxylic acids one
should search in the structure of the acid cation complex which is formed at acid protonation. Obviously, two proton-deficient neighboring centers with lone-pair electrons exist in an
acid molecule, therefore two structures are possible for the protonated complex. In the first
of them , a positive charge is concentrated on the carbon atom in the structure of the carbcation
; in the other structure it is concentrated on the oxygen atom in the structure of oxonium
ion: Deprotonation of the carbon atom with adsorption of the acid molecule on nickel fluoride at fluorination
will result in the formation of structure –C-O-F which is stabilized with fluorine monoxide
formation and carbonyl elimination in the form of CO or COF2. Deprotonation of
the oxygen atom may be accompanied with solvolysis with formation of appropriate acylfluoride
and hydroxonium cation followed by subsequent acylfluoride fluorination: If there is a second proton-deficient centre in the organic radical,for example an aromatic ring
or alkoxy group, repulsion of similar charges makes more preferable the formation of oxonium
ion whose positive charge is by a distance of C-O bond length further away than carbcation.
In this case acylfluoride formation becomes more easy and as a result yields of perfluoroacylfluorides
in fluorination of benzoic acid [97,98] and alkoxycarbonic acids [92] are higher than those
for typical carboxylic acids: Electrochemical fluorination of esters of carboxylic acids was succeeded in relatively high yields
of perfluoroacylfluorides. Thus, methyl butyrate fluorination [99] gave perfluorobutyl fluoride
in 35%mol yield, which is a little different in comparison with the results of butyryl
fluoride fluorination [91] and is in good agreement with the data of S. Nagase et al.[100]
given in the table Table![]()
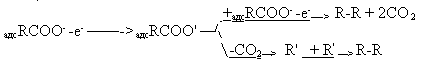
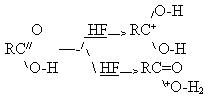
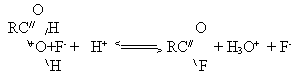
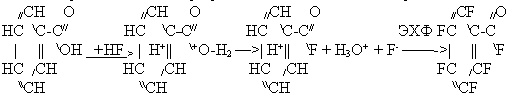
|
Starting substrate |
Yield, %mol |
||
| CF 3COF | CF 3CF2COF | CF 3CF 2CF 2COF | |
| CH3COOCH2CH3 |
52.3x 2 |
- |
- |
| CH3COOCH2CH2CH3 |
19 |
24 |
- |
| CH3COOCH2 CH2CH2CH3 |
22 |
1.0 |
15 |
| CH3CH2COOCH2CH3 |
20 |
23 |
- |
| CH3CH2COOCH2CH2CH3 |
2.1 |
33х2 |
- |
| CH3CH2COOCH2CH2CH2CH3 |
2.4 |
25 |
12 |
| CH3CH2 CH2COOCH2CH3 |
18 |
1.0 |
16 |
| CH3CH2CH2COOCH2CH2CH3 |
1.7 |
16 |
11 |
| CH3CH2CH2COOCH2CH2CH2CH3 |
2.3 |
2.0 |
28,5x2 |
The process of ester fluorination includes ester solvolysis with formation of acylfluoride and
an alcohol which after hydrogen substitution with fluorine at ![]() -carbon atom undergoes dehydrofluorination with formation
of acylfluoride group according to the scheme:
-carbon atom undergoes dehydrofluorination with formation
of acylfluoride group according to the scheme:
![]()
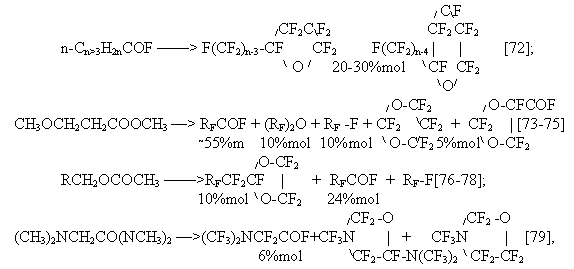
Simons and co-workers in fluorination of various oxygen-containing compounds such as alcohols [39], ethers [102], ketones [66] identified only fluorocarbons in the electrolysis products. Later Nagase and co-workers after broad investigations of ECF of oxygen-containing compounds showed that all of them form perfluoroacylfluorides as a result of electrolysis [67-69,100,101].
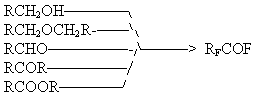
The dependence of perfluoroacylfluoride substance yield on the carbon chain length and the functional group is shown in Fig.6.
We mentioned above the differences in yields of perfluorinated compounds in dependence on their structure, when primary, secondary amines and pyridine are fluorinated with rupture of C-N bonds, whereas from aliphatic tertiary amines [102], N,N -dialkylanylines and cyclohexylamines [71,106,107] , salts of quaternary N-alkylammonia hetercyclic bases [108,109] there are produced perfluoro analogs with high yields. The dependence of perfluoroanalogs yield on the chain length of N-alkyl substituent according to data [106] is given in Fig.7.
Before we mentioned producing perfluoroalkansulfofluorides from conforming alkansulfohalogenides, but it is not complete data on ECF of sulfur-containing compounds. Electrolysis of solutions of dimethylsulfide and carbon disulfide in AHF results in the formation mainly of sulfur trifluoromethylpentafluoride in 57 and 90%mol yield [110]. A yield of sulfur bistrifluoromethyltetrafluoride did not exceed 5%mol. Further by ECF of dialkylsulfides and –disulfides, also cyclic thioethers [111] there were produced appropriate sulfur perfluoroalkylpentafluorides and sulfur bisperfluoroalkyltetrafluorides in good yields, moreover the yield of sulfur bisperfluoroalkyltetrafluorides increases up to that of pentafluorides with the chain growth .
Almost in all cases of ECF of partially fluorinated compounds ,yields of the electrolysis products increase considerably. We mentioned the fluorination of difluorocyclohexane [80] when the yield of perfluoro-C6 structures is noticeably higher than for hexane. Similarly:
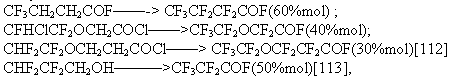
Using this advantage of partially fluorinated compounds, perfluoroenanthic and perfluoropelargonic acids by ECF of telomer alcohols of H(CF2CF2)nCH2OH formula where n=3,4 are produced in Russia.
(to be continued)
Fluorine Notes, 1999, 3, 3-4
