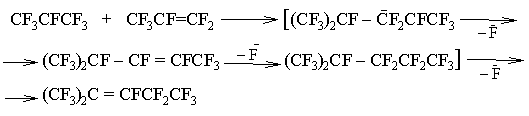Fluorine Notes, 1999, 3, 9-10
Hydrofluorination of perfluoroolefins with the terminal double bond.S.M. Igoumnov, A.A. Shipigusev, V.A.Soshin, G.I. Lekontseva, B.I. Drozhdin Perm department RNC "Applied chemistry" Perfluoroolefins hydrofluorination is the basis of ozone-safety refrigerants production technology. It is known that addition of electrophilic reagents to fluoroolefins is found to be difficult because the substitution of hydrogen atom at Sp2-hybrid carbon atom with fluorine brings to a reduction in the electron density both in p-system and in s-component of the multiple bond. The chemical consequence of that is a reduction in sensitivity of fluorinated olefins to electrophilic attack and a necessity to carry out such reactions at elevated temperatures using catalysts. As catalysts for these reactions there are used mostly derivatives of chromium and vanadium [1], chromium oxyfluoride [2], weakly base ion-exchange resins containing tertiary amino groups [3], antimony catalyst [4], tantalum and niobium pentafluoride [5], silver fluoride [6], copper sulfate applied on activated carbon among them [7]. But the existing methods do not provide high enough yield of fluoroalkanes and complete conversion of the starting fluoroolefin. Therefore it was of interest to make a search for effective and available catalysts for hydrofluorination providing a more complete conversion of fluoroolefin and high content of the main substance. As such catalysts, we proposed activated carbon promoted by fluorides of alkali metals KF, NaF, CsF and RbF. The hydrofluorination of fluoroolefins was carried out by hydrogen fluoride at a temperature of 250-450oC at a mole ratio of HF:olefin from 1:1 to 2:1 according to the scheme:
Obviously the process runs via the intermediate formation of the carbanion:
It should be noted that only perfluoro-(2-methyl-penten-2) is formed as a by-product under the reaction conditions, evidently as a result of isomerization:
During the investigation some regularities have been studied: the content of fluorides of alkali metals was changing from 0.05 to 0.5kg per 1 dm3 of the catalyst. Using lower quantities of fluorides leads to a decrease in the catalyst activity and an increase in the content of fluorides over 0.5 kg per 1 dm3 of the catalyst does not contribute to its activity increase. A decrease in the process temperature below 250oC brings to a decrease in conversion of fluoroolefins to goal products and when the temperature is increased to above 450oC, deactivation of the catalyst takes place. The mass flows of the reagents are selected so that to keep the mole ratio of HF:fluoroolefin within a range of 1:1 to 2:1. Feeding HF in a lower amount results in a significant reduction in the yield of the goal products and an increase in HF amount over the mole ratio of 2:1 is of no practical importance because does not give any further increase in the yield. The compounds obtained (I, II, III, IV) contain 99.5%min of the main substance. The yield is 97-99% of the theoretical one. Experimental A tubular reactor made of stainless steel of 50mm diameter and 560mm long of 1.1 dm3 capacity provided with electrical heating, a thermocouple pocket and branch pipes for input of the starting components and output of the reaction products, is filled with 1.0 dm3 of catalyst. The starting fluoroolefin is passed through the catalyst heated to 380oC at a rate of 120-150g/hour and anhydrous hydrogen fluorine is added at a rate of 17-30g/hour. The gases leaving the reactor are neutralized with 20% aqueous solution of potassium or sodium hydroxide , passed through a column filled with a lime chemical adsorbent and condensed in a trap cooled to a temperature of minus 40oC. The reaction products were identified by means of IR- and NMR-spectroscopy. Conclusions.
References
|
Fluorine Notes, 1999, 3, 9-10