Fluorine Notes, 1999, 2, 1-2
 |
Vsevolod V. Berenblit,
Ph.D., Leading Scientist of Laboratory of Fluorganic Polymers. S.V. Lebedev Synthetic Rubber Research Institute. |
Electrochemical fluorination of organic compounds.
Introduction
Any process of organic fluorination which necessarily includes substitution of hydrogen or any other atom in the chain of an organic substrate with fluorine is a reduction-oxidation process because fluorine as the group VII element of the second period from Mendeleyev's periodic system possesses a maximum possible electron affinity (4.15eV) [1] and a maximum standard potential of the redox reaction
F 2+2e- < = = > 2F* , E 0 = 2.87V [2]
And hence fluorine oxidizes C-X bond (where X=H in particular) to C-F bond. The reduction-oxidation potential of the reaction is known to be an electric measure of free energy change according to Gibbs and is connected with the latter by the equation
D F
=-z FE,
Where z is a number of electrons in the redox reaction and F is Faraday.
Of course, introduction of fluorine as a substituent in the organic structure results in formation of the most oxidized form of the given organic compound on condition of its original carbon chain preservation.
But because the energy of C- F bond evaluated as 4.6-5.2 eV [3] in dependence on the structure is considerably higher than the energy of C-C bonds in the carbon chain, fluorination by elemental fluorine is usually accompanied with carbon skeleton destruction with formation of the end oxidized form of tetrafluoromethane.
A reduction in fluorine oxidation potential according to the Nernst's equation can be attained by means of fluorine dilution with an inert gas and temperature reduction, in some cases that provides fluorination of the organic compound without full destruction of the carbon skeleton.
Another method of such reduction is a use of higher metal fluorides of variable valence [4] as fluorine carriers; their redox potential is noticeably lower than that for elemental fluorine.
Fluorination of organic compounds can be made significantly easier by means of increasing the reduction-oxidation potential of the compound subjected to fluorination when preliminarily oxidized (primarily halogenated) starting compounds are used in the reactions. Just from these processes the chemistry and technology of organofluorine compounds began in the 1930-40 years, especially freons of the methane and ethane series and traditional fluoro monomers such as di-, tri- and tetrafluoroethylenes, trifluoro- and hexafluoropropenes.
An electrochemical system of anode/electrolyte/cathode is distinguished from a typical reduction-oxidation system by a space separation of oxidation anode and reduction cathode components of the redox reaction. When an external source of direct current is used, such a system allows (within the limits of stability of the given electrolytic medium) to regulate smoothly and to keep to a high precision the necessary value of the oxidation anodic potential and the cathode reduction potential.
The voltage on the electrodes in such a system U is added from:
U= Eai + Eci+JR,
Where Eai is the anodic potential at the current density i; Eci is the cathode potential accordingly measured with respect to the nonpolarizible reference electrode; JR is the ohmic voltage drop; J is the electrolysis current; R is the resistance of the electrolyzer. The potential of each electrode Eei consists of:
Eei=E+ ![]()
Where E is the equilibrium potential of the electrode reaction; ![]() - is the overvoltage of the electrode reaction.
- is the overvoltage of the electrode reaction.
The equilibrium potential depends on the concentration according to Nernst's equation:
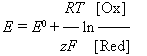
The overvoltage depends on the current density according to the Tafel's equation:
h = a + blogi
Having set the concentration and providing the activation energies of the electrode processes by means of the overvoltage determined by b value, one can get a possibility to set the necessary rate of the anode and cathode processes with the appropriate current densities by varying the current in the external chain and the surface area of the every electrode.
A process of anode hydrogen substitution with fluorine was carried out for the first timein the 1927 when Lebeau and Damiens [5], studying the electrolysis of KF*3HF melt in the presence of moisture on Nickel anodes, found up to 30% of fluorine monoxide OF2 in the electrolysis gases. They attributed its formation to elemental fluorine interaction with water at washing of the electrolysis gases from hydrogen fluoride. But Ruff and Mensel [6] showed that fluorine reacts very slowly with water and hence the authors observed the water electrochemical fluorination. Later Ruff and co-workers [7] identified nitrogen fluoride and trifluoride in the electrolysis of NH4F*HF melt on Nickel whereas more recently Lebeau and Damiens [8] had found C-F bond formation in the electrolysis of fluoroberyllates of alkali metals on carbon anodes having separated perfluoromethane from the electrolysis gases.
But only 20 years later after Manhattan project had announced its demand in organofluorine products,
Simons [9] developed electrochemical fluorination of organic compounds on a nickel anode in a medium
of anhydrous hydrogen fluoride (AHF) at a temperature below the electrolyte boiling point and at
an electrode voltage preventing fluorine evolution. Very soon this process merited universal acknowledgement
and got industrial application as practically the sole (at least for that time) method of synthesis
of functional derivatives of fluorocarbons, especially synthesis of perfluorocarboxylic acids and
their different derivatives, perfluoroalkan sulfonic acids etc.. Already in the 1950-s at facilities
of 3M company in the USA an industrial plant with the current load of 10 000A[10] was commercialized.
Fig.1 presents the construction of the electrolyzer and the electrode
package of Simons’ design, which was used in our investigations in particular.


Fig.1 Laboratory electrolyzer and electrode pack for Simons’ ECF process
Until now the process of electrochemical fluorination has attracted attention of researchers from different countries. Teams of researchers involved in ECF have worked in the USA, Great Britain, Russia, Italy, Japan, China, Germany, Czechia, Armenia, and South Africa. The number of publications concerning ECF according to Simons is not decreasing from year to year and has reached 5-10 original articles and patents per year.
Later Knuniants and Rozhkov [11] discovered a possibility of partial fluorination of aromatic compounds on a platinum anode at potentials below the standard potential of fluorine evolution in an acetonitrile medium with bifluorides of quaternary ammonia bases as an electrolyte by means of aromatic carbcation formation on the anode at oxidizing the substrate with subsequent addition of fluorine anion. Shmidts [12] used the similar mechanism of electrochemical fluorination of the double bond in anhydrous acetic acid. But application of this ECF method is obviously limited by the field of pharmaceutical industry only.
Also, there has been developed a high-temperature CAVE/Phillips process of ECF[13], that is electrolysis on a porous anode of amorphous carbon and a steel cathode in the elctrolyte of KF*2HF melt at 95oC with feeding the organic substrate (mainly hydrocarbons) into the electrolyzer through the anode pores. When the standard potential of fluorine evolution on the anode surface is attained, the formation of a surface layer of polycarbon monofluoride (CF)n in the pores begins. It is known, at fluorine interaction with flaked graphite structure, polycarbon monofluoride is formed as a white powder, whereas in case of amorphus carbon it (polycarbon monofluoride) is preserved on the surface as a passive film which prevents wetting the carbon surface by the electrolyte and provides organic substrate introduction into the electrolyzer through the pores. In the pores on the interface there occurs a discharge of the fluorine containing anion with fluorine radical formation which reacts directly with the organic substrate and partially or fully fluorinated products are produced in dependence on the feeding rate or the number of substrate passing cycles through the pores. Fig.2 exhibits the construction of Phillips' design electrolyser. But up to now application of CAVE method on an industrial scale is limited by the absence of porous amorphous anodes provided industrial current load.

Fig.2 laboratory electrolyzer for CAVE Phillips ECF process
As mentioned above, electrochemical systems allow carrying out redox processes within the limits of stability of the given electrolytic medium. Thus, for water these limits are restricted thermodynamically in anode region by the standard potential of oxygen evolution according to the reaction:
2 H2O-4e- < = = => O2 + 4H+ , E0 = 1.229 - 0.059 pH V[2]
and the standard potential of hydrogen evolution according to the reaction:
2H+ + 2e- < == >H2 , E0 =-0.059 pH V[2]
Practically this potential difference can be expanded to the cathode region by a value of constant a in Tafel's equation by means of electrolysis on the cathodes with a high hydrogen evolution overvoltage and it may be expanded to the anode region by means of oxygen evolution overvoltage and specific adsorption of the volume anions displacing water dipole molecules from the double electric layer at high anodic potentials.
It is known that fluorine anion does not possess an ability to be specifically adsorbed in the anode region and is not able to displace water from the double electric layer, therefore one fails to realize the potential of elemental fluorine evolution and oxidation of organic molecules with subsequent ionic fluorination in an aqueous medium.
In connection with this, media comforming to the necessary limits of the anodic potentials for ECF are anhydrous hydrogen fluoride, in which in 1986 Moissan [14] evoluted elemental fluorine by electrolysis on a platinum anode, and its complexes with fluorides of alkali metals which are widely used in the modern technology of fluorine production by means of electrolysis on nongraphite anodes [15]. The overvoltage of fluorine evolution is very high and the constants from the Tafel's equation attain values of 4V (for a) and 0.7 ( for b).
|
to be continued |
Fluorine Notes, 1999, 2, 1-2
