Fluorine Notes, 1999, 2, 9-10
Biocide activities of perfluorosubstituted azines and azoles.
Popova,L.M.; Trishina,A.Yu.; *Vershilov, S.V.; Nianikova, G.G.;Pashkov.P.A.
Saint-Petersburg State Technological Institute, 26 Moscovsky ave., St.-Petersburg, 198013 Russia;
*Russian Scientific Center " Applied Chemistry" 14, Dobrolubov ave., St.-Petersburg, 197198, Russia
A problem of biological damages of building materials and natural objects comprises a wide range of scientific and technical problems associated with protection from aggressive media. The chemical protection from biological damages is connected with use of biologically active substances (either as individual substances or as components of compositions) able to prevent evolution of organisms, to weaken or destroy organisms causing damages of materials[1,2]. A certain attitude to these protection means (biocides) have fungicides, bactericides, insecticides, algicides etc.
There has been investigated the biocide activity of the series of perfluorosubstituted derivatives of pyrimidine [3] and 1,2,4-triazole, also predecessors of azoles [4,5]: perfluoroacylthiosemicarbazide and perfluoroacylaminoguane. Determination of bactericide activity of the substances produced was carried out by a method of diffusion into agar. The method has been described in detail in literature [6,7] and based on the ability of investigated substances to diffuse in agar media and form zones in which test cultures of microorganisms did not develop [6]. The difference between this method and others is its relative simplicity and possibility to obtain express estimation of biological activity of the compounds synthesized.
Representatives of different taxonomic groups were chosen as test-cultures: fungi Aspergillus niger and Penicillium crysogenum, Trichderma viride, gram-negative bacteria Serratia marcescens, yeast Sacchoromyces cervisiae, Chaetomium globosum.
Compound (II d) exhibited a good result with respect to Serratia marcescens, the rest of the compounds showed weak biocide activity.
Biological testing has been carried out with respect to mucous bacillus Bacillus musilaginosus destroying actively construction materials of hydraulic structure [2].
According to modern conception, the process mechanism of biologically active compounds includes two stages: transportation to the site of actions and interaction with cell structures [8,9]. The action of the compound is mostly determined by its hydrophobic properties in the first stage and by its steric and electronic characteristics in the second stage. A possible action mechanism may consider membranotropic activity of derivatives of perfluoropyrimidines associated with high lipophilic nature of the perfluorinated molecular fragment.
The investigation has discovered that fluoropyrimidines with mercapto- and methyl- groups in position 2 of the ring (III a,b, and V a,d,e respectively), in position 5 of triazole ring(VI c, VII a,c) and perfluoroacylthiosemicarbazides (VIII a, b,d) possess the greatest bacteriostatic activity with respect to Bacillus musilaginosus.
Probably, the biostatic activity depends on the presence of sulfur atom. The length and composition of the perfluorinated radical influence the activity of substances. Compounds (V d,e, VII c, VIII a,c,d) have an optimal length of heteroalkyl substituent (C = 6-8) and due to the presence of methylthio- group the activity of these compounds is the greatest among all the tested compounds.
A significant increase in the length of perfluorinated radical leads to extinction of bacteriostatic activity. In this connection compounds ( I E, II E, III E, V E) possess a feebly marked activity.
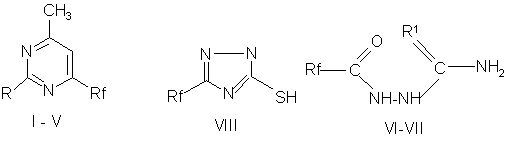
Rf= a C6F13 , b- C8F17 , c - CF(CF3)OC3F7 , d - CF(CF3)[OCF2CF(CF3)]2 , e- CF(CF3)[OCF2(CF3)]nOC3F7 (n=9)
R= NH2(I) , OH (II), SH (III) , NH(n-Bu) (IV), SCH3 (V)
R1= NH (VI), S (VII)
6-perfluorosubstituted pyrimidines (I -V) have bacteriostatic activity comparable with that of perfluoroacylhydrazines (VI -VII) and 3-perfluorosubstituted 1,2,4-triazoles (VIII).
To evaluate quantitatively biological activity of the compounds investigated on the base of experimental data, we assume exponential dependence of the dimensionless diameter of the zone of growth suppression of microorganisms ( d) on the substance concentration (c).
So, it follows that
![]()
α and ![]() were determined by a graphical method. The results of calculations are given in the table .
were determined by a graphical method. The results of calculations are given in the table .
Table. Results of the mathematically processed experimental data.
| Compounds |
|
α |
|
| IIIa | -1,92 | 0,0170 | 2,5 |
| Va | -2,40 | 0,0160 | 1,50 |
| Vd | -1,60 | 0,0130 | 2,62 |
| Ve | -1,11 | 0,0090 | 3,10 |
| VIIIc | -1,39 |
0,0080 |
2,60 |
| VIIIe | -1,35 | 0,0105 | 2,70 |
Let us introduce a concept of biological activity of substances independent of the concentration of the
substances. We accept this parameter as equal to the tangent of the angle between the tangent slope
and the experimental curve at infinitely small concentration, that is equal to the derivative of
function ![]() (c)
at c =0.
(c)
at c =0.
Hence,
![]()
So, the quantitative characteristic of biological substance activity (![]() ) independent of the compound concentration and characterizing individual
properties of the product has been introduced.
) independent of the compound concentration and characterizing individual
properties of the product has been introduced.
The functional dependence of the dimensionless diameter of the zone of growth suppression of bacteria
culture (![]() ) on the concentration and biological activity (
) on the concentration and biological activity (![]() ) has been determined.
) has been determined.
References
1. Biopovrezhdeniya v stroitel'stve / Pod red. F.M. Ivanova‚ S.N. Gorshina. - M.: Strojizdat, 1984. - 320 s.
2. G.G. Nyanikova, E.Ya. Vinogradov // Sb. Aktual'n. vopr. khim. nauki i tekhnologii, ehkologii v khimpromyshlennosti, M.: AO NIITEHKHIM, 1995, V. 3, s. 1-17.
3. L.M. Popova, A.U. Trishina, S.V. Vershilov, A.I. Ginak, B.N. Maksimov // J. Fluorine Chem. - 1998. (N 3963, p. 1-8.)
4. S.V. Vershilov‚ L.M‚ Popova, V.E. Mungalov‚ N‚A. Ryabinin // Zhurn. org. khimii, 1994, t. 30, - v. 8, s. 1241-1244.
5. S.V. Vershilov, L.M. Popova, V.E. Mungalov‚ N‚A. Ryabinin // Zhurn. prikl. khimii, 1994, t. 67, v. 7, s. 1124-1126.
6. Spravochnik po mikrobiologicheskim i virusologicheskim metodam issledovaniya / Pod. red. M.O. Birgera.-M.: Meditsina, 1982, 464s.
7. Brian L.E. Bakterial'naya rezistentnost' i chuvstvitel'nost' k himiopreparatam.- M.: Meditsina, 1984, 272 s.
8. Barenbojm G.M‚‚ Malenkov A.G. Biologicheski aktivnye veshchestva.Novye printsipy poiska. - M.: Nauka, 1986, 362 s.
9. Landau M.A. Molekulyarnaya priroda otdel'nyh fiziologicheskih protsessov.-M.: Nauka, 1985, 260 s.
10. Spravochnik po matematike / Pod red. I.N.
Bronshtejna, K.A. Semendyaeva. - M.: Nauka, 1980, 976
Fluorine Notes, 1999, 2, 9-10
