Fluorine Notes, 1999, 2, 7-8
 |
L.M. Popova, A.Yu.Trishina, S.V.Vershilov. |
 |
Synthesis and properties of new perfluoro-S-triazole [1, 5-a] pyrimidines.
Syntheses of derivatives of 1, 3- diazines with a great number of perfluorinated links containing a functional group in addition to inert perfluorosubstituent in the structure are of great importance for creation of novel products possessing a wide spectrum of biological and surface - active properties.
Substituted derivatives of pyrimidine are valuable Intermediate products for the formation of condensed heterocyclic systems, specifically triazolopyrimidines exhibiting pharmacological properties (1). Triazolopyrimidines are known also to be used as effective herbicides (2, 3). Earlier we reported the synthesis of 7-methyl-5-perfluorohexyl-5-triazolo [1, 5-a]pyrimidine (4).
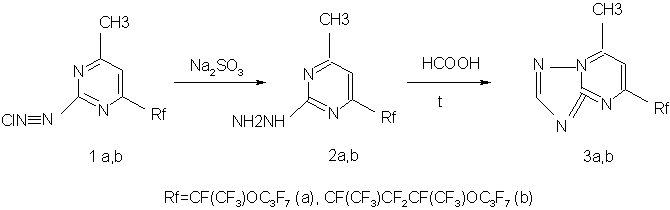
To continue investigations on synthesis and properties of derivatives of azines, diazotization of 6-perfluoro-2-amino-4-methylpyrimidines
was carried out. Products of diazotization (1a, b) are stable in aprotic solvents such as fluorocarbons.
In IRS there was observed a band of valence vibrations
n N![]() N at 2210-2360 cm-1.
N at 2210-2360 cm-1.
By reduction of 4-methyl-6-perfluoro pyrimidyl-2-dlazonium chlorides (1a,b) by sodium sulfite in a water-alcohol solution (Fisher reaction), the appropriate 2-hydrazinopyrimidines (2a,b) were produced. Subsequent boiling compounds (2a,b) in formic acid results in the formation of new group of 5-perfluoro 7-methyl-s-triazolo[1,5-a] pyrimidines (3a,b) stem from rearrangement (similar to the Dimroth rearrangement) with perfluoro fragment in the pyrimidine part of the condensed cycle:
Experimental.
Synthesis of 4-methyl-6-perfluoro pyrimidyl-2-diazonium chlorides (1a,b). A solution of 1.02g (0.014 mole) of NaNO2 in 7 mL of water was added dropwise to 0.014 mole of 6-perfluoro 2-amino-4-methylpyrimidine [5] in mixture of 15 mL of isopropyl alcohol and 6.5 mL of HCL at 0-2oC during 2 hours at vigorous stirring. The product was extracted with R-113 and washed with water to neutral reaction.
6-perfluoro 2-hydrazino-4-methylpyrimidines (2a,b). 0.025 mole of alcohol solution of 6-perfluoro 4-methyl-2-pyrimidyldiazonium chloride (1 a,b) was added dropwise to a solution of 5.9g (0.05mole) of sodium sulfite in 20 mL of water at 0oC, 19 ml of HCL (concentrated) was added and the mixture was aged during 12 hours. Then 8 mL of HCL (concentrated) was added and the reaction mixture was boiled during 3 hours. Then the reaction mixture was neutralized by alkali solution to pH 7-8, extracted with a mixture of R-113 and ethylacetate, saulted out with NaCI, dried and the solvent was distilled. Products (2a,b), oily substances of brown color, yielded within a range of 67-70%. IRS, thin layer (cm-1): 3500, 3320, 3170,1680, 1610, 1410, 1350-1000.
5-perfluoro 7-methyl-5-triazolo[1,5-a] pyrimidines (3a,b). 4.8 g (0.1 mole) of formic acid was added to 0.01 mole of 6-perfluoro 2-hydraano-4-methylpyrimidine (2a,b), the reaction mixture was boiled during 12 hours, then it was washed with water, extracted with ethylacetate ( 3x10 mL), dried with MgSO4 and the solvent was evaporated. The products (amorphous masses) yielded in 60 and 65% respectively . Data of IRS are presented in the table.
Data of IRS of 5-perfluoro 7-methyl-s-triazolo[1,5-a] pyrimidines (3a,b):
|
Compound |
Wave number.cm-1 |
|
3a |
3130, 2940, 2910, 2310, 1620, 1545, 1440,1370-980 |
|
3b |
3120, 2995 ,2910, 2310, 1620, 1515, 1440, 1370-950 |
References
1. Patrik Т.В. J.Chem. 1979. V. 56, N 4. P. 228-230.
2. Patent US 526238, Herbicidal Compositions with Increased Crop Safety / Noveroske R.L., Dow Elanco (England).
3. Patent US 4904301, 5-Fluoromethyl-l,2,4-Triazolo[l,5-a]-Pyrimidines / Pearson N.R., Kleschick W.A., Carsoc C.M. (The Dow Chemical Co).
4. A.Yu. Trishina, L.M. Popova, S.V. Vershilov, N.A. Ryabinjn, A.I. Ginak / Abstr. 2nd Intern. Conf. of Chemistry, Technology and Application ofFluorocompounds in Industry, September 23-26, 1997. St. Petersburg, Russia, 1997. P. 126-127
5. A.Yu. Trishina, L.M. Popova, E.V. Irisova, S.V. Vershilov, S.V. Konyuhova‚ A.I. Ginak / Khimiya azotistyh geterotsiklov: Tez. dokl. mezhinst. koll., 18 oktyabrya 1995 g. -Chernogolovka, 1995. -S. 96.
Fluorine Notes, 1999, 2, 7-8
