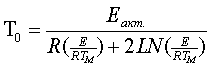Investigation of the thermal stability of perfluoropolyethers by method of thermal analysis.T.G.Tiunova*, A.V. Tiunov**, F.M. Moukhametshin*** *Institute of technical chemistry, dpt. of Russian Academy of Science **JV "Bytkhim" *** RSC " Applied Chemistry", Perm branch Perfluoropolyethers (PFPE) have attracted attention by their unique properties that allow to solve many problems arising in modern technique. They are used as thermally and chemically stable liquids, oils and bases for plastic lubricants [1]. PFPE of hexafluorooxypropylene structure ( of Fomblin type) are used as dispersion medium of plastic lubricants efficient in a wide temperature range. Thermal stability is one of the most important requirements to lubricants [2,3]. To evaluate thermal stability, thermal methods of analysis are used, derivatography in particular [4]. Sianesi [5] at investigation of Fomblin destruction has established that its thermal stability is determined by the presence of metals and their compounds and does not practically depend on the oxygen presence. PFPEs of difluorooxymethylene structure (POM) are efficient at a lower temperature than the working temperature of PFPE of Fomblin type. But no reference data on POM thermal stability has been found. The aim of this work was to study thermal stability of POM liquid in different materials. In this paper the results of the study of thermal stability of POM liquid of the general formula CF3O(CF2CF2O)n(CF2O)mCF3, where
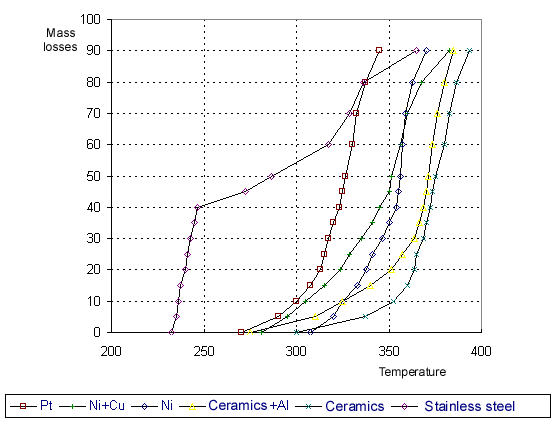
Derivatograms have been obtained by means of derivatograph Q-1000 "D" of " MOM' made of Paulic-Paulic-Arday system. Experiments were carried out in an air medium in a temperature range of 20oC - 500oC at a rate of temperature increase of 5 degree/min. The sensitivity for the curves was: 1/3mV for DTA, 1/10mV for DTG. Calcined aluminum oxide (Al2O3) was used as a standard. There were studied POM samples of molecular mass of 6000. Programmed heating was carried out in crucibles of different materials: nickel, stainless steel of 18Н12Х10Т type, ceramics and platinum. Derivatograms for the reference substance were taken in self-made crucibles of nickel and stainless steel. These derivatograms are absolutely identical to those obtained in standard ceramic and platinum crucibles from the derivatograph set. The curves of the mass loss of POM liquid versus the temperature are given in Fig.1 which shows that the thermal stability of POM depends on the material of the crucible which was used for testing. Fig.1 : Dynamical thermogravimetric analysis of POM
The following values were taken as characterizing parameters for thermal stability: a temperature at which the product loses 5% of the starting mass [3], an integral temperature corresponding to 50% mass loss [6] and a temperature of To which was calculated according to the formula [7]:
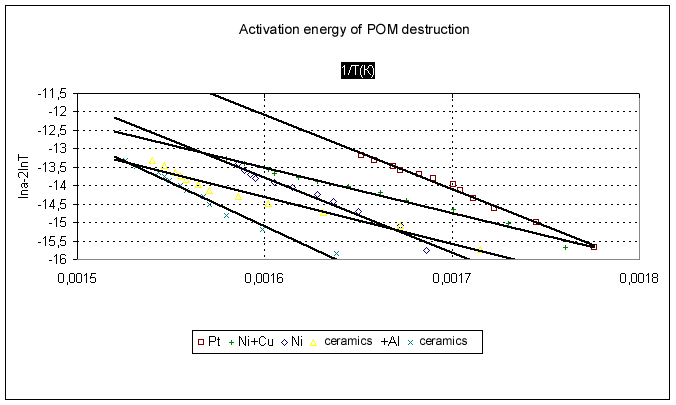
where E is activation energy (J/mol) , Tm is the temperature of the maximum rate of structure decay (which was determined by projection of the top of the corresponding peak of the thermogravitometric curve derivative to the temperature curve), R is the universal gas constant. The percentage loss was determined according to the thermogravitometric curve of the mass loss versus the temperature of heating. To evaluate reproducibility of the experimental results, POM derivatograms were taken thrice under the same conditions. The absolute divergences of the received values on thermal stability were within +5oC. Liquid POM is possible to be used in nickel equipment with copper gaskets, therefore there was carried out POM testing in a nickel crucible with addition of copper wire of M1 type. The weight ratio of POM/copper was 8/1. Influence of aluminum on POM thermal stability was studied in a ceramic crucible with addition of aluminum in the ratio of 17/1. The results obtained are given in the table. The activation energy of the destruction process is calculated according to a method proposed by Piloyan [8]. Fig.2 presents a linear dependence that points to Arrhenius dependence observance and to the correct study of POM destruction under nonisothermal conditions. Fig.2.
The analysis of the data obtained has shown that the highest thermal stability of POM is observed in the absence of metals ( see the table). Table. Effect of the material of a crucible on POM thermal stability.
The results obtained ( the table, Fig.1 and Fig.2) allow to conclude that aluminum introduction affects thermal oxidizing stability and the rate of mass loss. The temperature of the beginning of POM destruction at aluminum introduction goes down by approximately 90 degrees. Probably the reduction in the decomposition temperature is related to participation of aluminum oxides on the metal surface in the process of POM destruction. It is known [2] that reactive centers of anion character arise in aluminum oxide at heating . They can react with ether bonds (-C-O-) of POM strongly reducing the energy of rupture of these bonds at the same time. Indeed, the activation energy of thermal oxidizing destruction of POM in a ceramic crucible is 197 kJ/mole against 106 kJ/mole in the presence of aluminum. Other metals also reduce the temperature of the beginning of POM destruction. The lowest thermal stability is observed at using stainless steel. The stainless steel of 18H12X10T type contains 12% of Cr, 18% of Ni, 10% of Ti, 60% of Fe. The formation of various oxides is possible on the surface of a crucible of stainless steel. It is known from literature [1] that ferric oxide Fe2O3 and titanium dioxide TiO2 are strong acids of aprotic and protic type able to donor-acceptor interaction with ether groups. Probably this donor-acceptor interaction shifts the temperature of the destruction beginning to the direction of lower temperatures more than by 100 degrees. There is observed a reduction in POM thermal stability at using crucibles of nickel and platinum in comparison with ceramics. The rate of POM destruction in the nickel crucible with copper addition rises , that results in thermal stability reduction by 86o in comparison with nickel. Apparently that is connected with the formation of oxide film on the metal surface which catalyzes POM decomposition [2]. We have found out in this work that the POM thermal stability in dependence on the material used decreases according to the following sequence: ceramics>Ni>PT>Al> Ni+Cu>stainless steel. The effect of metals on POM thermal oxidizing stability has been determined . The activation energy of POM destruction correlates with the temperature of decomposition beginning. On ground of the data obtained it is possible to draw a conclusion that the presence of metal interacting with the oxygen atom of difluoromethylenoxide chain (-CF2OCF2-) determines the kinetic parameters of the process of polymer thermal destruction.
References
|
|||||||||||||||||||||||||||||||||||||||||||||||||