 |
V. Andriushin and N. Pavlova |

|
 |
V. Andriushin and N. Pavlova |

|
Report 2.
1. Introduction
It is well-known (1) that high reactivity of oxiranes is determined by the structure of three-membered ring and high angular strain of the molecule together with its torsion strain caused by screening interactions. Tetrafluoroethylene oxide (TFEO) - is the less stable and the most reactive representative of the homologous series of terminal oxides of perfluoroolefines.
The present paper reviews and systemizes the available data on TFEO stability, reactivity and conditions of its polimerization, besides some properties and reactions of TFEO are given rather briefly because its detailed analysis has been published in review (2) and study (3).
2. Structure of tetrafluoroethylene oxide
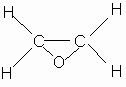
Tetrafluoroethylene oxide is a gas with a boiling temperature of -63.5oC. An investigation of TFEO microwave spectra allowed to determine molecule structure sufficiently reliably (4,5). Below there are given the angles and lengths of bonds in the molecules of TFEO and its nonfluorinated analog.
Molecular formula |
C2F4O |
C2H4O |
Molecular mass |
116.015 |
44.053 |
Angles: C C O F C F |
59o17' 109 o 01' |
61 o 24' - |
| Bond lengths, A o: C -O C –C C –F |
1.391 1.426 1.329 |
1.44 1.47 - |
Structural formula |
 |
 |
Dipole moment |
0.576 D |
1.8 – 1.9 D |
As it is seen from the above presented data, the lengths of bonds C-C and C-O in TFEO molecule are shorter than those in a molecule of ethylene oxide, that points to a larger ring strain . The presence of fluorine atoms, strong electron seeking substituents, in the TFEO molecule reduces considerably the electron density of oxygen atom, that affects the value of the dipole moment which is 3 times less than the dipole moment of ethylene oxide.
The difference in the structure of TFEO and ethylene oxide determines also their different reactivity. In paper (6) there has been made a conclusion about TFEO molecule genotoxicity based on the data about the structure and properties of the TFEO molecule. As reactivities of ethylene oxide and TFEO are absolutely different, this paper considers only properties and reactions of TFEO.
3.Isomerization and thermal stability of TFEO.
Due to the ring strain, the TFEO molecule is distinguished by very low thermal stability, so it is better to keep it pure at a temperature below minus 78 oC, though that causes some difficulties in work with it. A great number of investigations has been devoted to TFEO conversion under different conditions but it seems impossible to determine clearly the effect of these or those factors on the TFEO molecule stability in the presence of different admixtures .
TFEO is known to be subjected either to isomerization to perfluoroacetylfluoride or to decomposition with formation of tetrafluoroethylene and perfluorocyclopropane.
Obviously, at a temperature of up to 40-45 oC there is only TFEO isomerization (12,17,18), besides this process is catalyzed by different admixtures, especially by hydrogen fluoride, and isomerization is passing rather easily even at a temperature of dry ice in the presence of hydrogen fluoride traces (7,8)
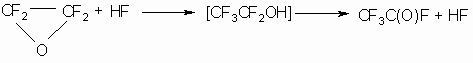
At a temperature above 140 o C, TFEO decomposition takes place according to the fundamentally different scheme(9,10):
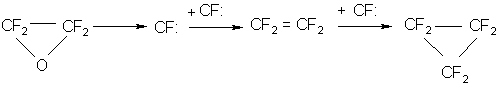
That is confirmed by the presence of tetrafluoroethylene, perfluorocyclopropane and carbonylfluoride in the products of TFEO decomposition.
Within a temperature range of 45-115 oC there is observed a gradual transition of TFEO isomerization to decomposition as it is seen from the data of Lenzi and Mele (9):
| Temperature, o C | 40 | 50 | 80 | 118 |
| Isomerization share,% | 100 | 95 | 60 | 0 |
| Decomposition share,% | 0 | 5 | 40 | 100 |
Thermal stability of TFEO depends on the purity of the product. So, at isomerization of the pure TFEO at 50 oC, there is observed a significant induction period which disappears in the presence of hydrogen fluoride or different sources of fluoride ion. In this case the induction period practically does not depend on admixtures of carbonylfluoride and perfluoroacetyl fluoride, that confirms the absence of autocatalysis process. For pure TFEO the dependence of the induction period on the temperature is described by the equation:
J (sec)= 10-6exp (16900/RT)
Catalytic activity in the reaction of TFEO isomerization is shown by fluorides of some metals on activated carbon, silica gels, zeolites, aluminum oxide promoted by SbF5 or by fluorine-containing compounds, also by walls of reaction vessels. Lewis acids do not act as catalysts in the process of TFEO isomerization. So, at interaction with boron trifluoride, the isomerization occurs at 50 oC(8). TFEO decomposition is an endothermic process, which may be accompanied with an explosion and is described by the equation:
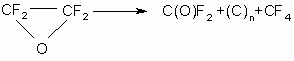
4. Tetrafluoroethylene oxide interaction with nucleophilic reagents.
In contrast to its hydrocarbon analog, TFEO according to a specificity of its structure is characterized only by reactions with nucleophilic agents. Interaction with electrophilic agents is not easy due to the influence of fluorine atoms possessing high electronegativity (2).
There were studied TFEO reactions with nucleophilic reagents of different classes such as alcohols, amines, ethers, nitriles, mercaptides, fluoroanhydrydes, some salts etc. Papers (7,14) describe TFEO interaction with water, ethanole and aniline with formation of oxalic acid and its derivatives: diethyloxalate and dianilide of oxalic acid respectively according to the scheme:
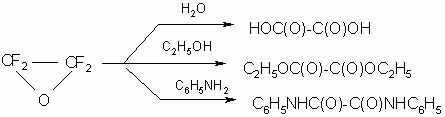
Later it was found that the reaction of TFEO with ethanol is not so clear and depends on the experimental conditions.
Together with the described above, according to our data, the etherification takes place as follows:
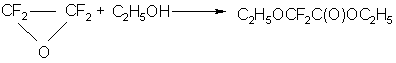
In reactions with ethers (15,16) TFEO forms both diethers and products of telomerization with n =1-20:
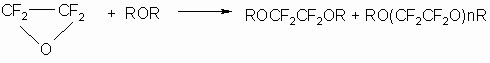
where R is alkyl with 2-6 carbon atoms.
The reaction with triethanolphosphate passes more complexly as follows (7):
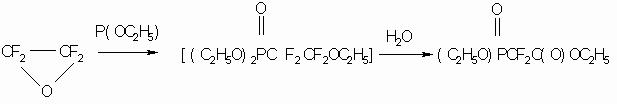
TFEO reaction with mercaptides was studied considering sodium phenylmercaptide (7), where phenyl ether of difluorothiophenylthiooxalic acid was received as a result:
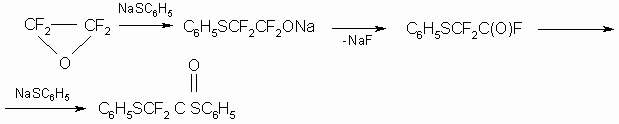
No interactions were obsrved with other salts: calcium carbonate and butyllithium,
while perfluoropropylenoxide , the next homologous representative of ![]() -oxides , reacts with butyllithium:
-oxides , reacts with butyllithium:
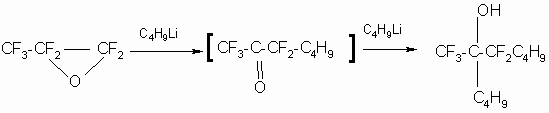
The reaction of TFEO addition to organic nitriles to form fluorine-containing heterocycles such as 2- oxazolines(17) is of interest:
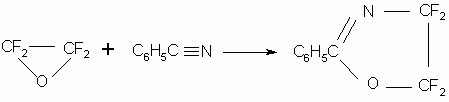
TFEO interaction with perfluoroalkoxides of alkali metals results in formation of
stable ester derivatives only when there is no fluorine atom at ![]() -alkoxide carbon atom , for example the interaction
with potassium pentafluorophenoxide:
-alkoxide carbon atom , for example the interaction
with potassium pentafluorophenoxide:
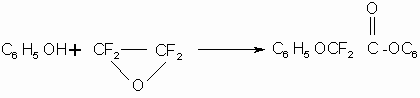
While analogous esters containing fluorine at alkoxide ![]() -carbon atom are not stable especially in the presence of
fluoride-ion (27-29).
-carbon atom are not stable especially in the presence of
fluoride-ion (27-29).
But for chemistry and technology of TFEO derivatives, there is of great interest the
reaction with fluoroalkoxy anions which are formed as a result of fluoride-ion action on
perfluorinated ![]() -oxides,
fluoroanhydrides of perfluorocarbonic acids, ketones (2, 19-23), for example:
-oxides,
fluoroanhydrides of perfluorocarbonic acids, ketones (2, 19-23), for example:
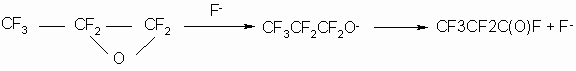
TFEO homopolymerization proceeds via a stage of fluoroalkoxy anion formation similar to the TFEO reaction with anhydrides, fluoroanhydrides and difluoroanhydrides of perfluorocarbonic acids, with ketones, lactones etc. (23 -30)
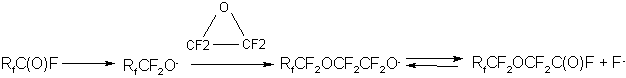
where R is the perfluoroalkyl radical
TFEO addition to difluoroanhydrides of perfluorodicarbonic acids proceeds preferably with participation of one fluoroanhydride group of perfluorodiacylfluoride to form appropriate perfluoroxycarbonic acids (28,29) FOC(CF2)nO(CF2CF2O)mCF2COF where n = 2-5, m = 0-3.
Products of analogous structure were produced when TFEO reacted with anhydrides of perfluorodicarbonic acids , lactones (27,28).